 |
in San Francisco, April 1 - 5, 2006   |
|
 |
in San Francisco, April 1 - 5, 2006   |
|
University
of Delaware undergraduate researchers live up to their national
reputation.

Front
Row left to right: Eric Hardter, Agata Bielska, Jessica hall,
Kristen Reese, Liang-I Kang, and James Kuczmarski
Back Row left to right: Kellie Machlus, Alfred Smith, Andrew Farach,
James Kelleher, Madeline Gregorits, Vivek Patel, and Patricia Hansen.
(Brett Hensley not shown) Recipients of Honorable Mentions in the ASBMB
Undergraduate Poster Competition are holding certificates. This
year UD students received more awards in this competition than students
from any other school in the country.
| Prof. Hal White Chem
& Biochem Prof. David Usher Biol Sci Prof. Gary Laverty, Biol. Sci. Dr. Seung Hong, Biol Sci Nicole Barkley*, Biol Sci |
Agata Bielska, Chem & Biochem Karla Boyd*, Biological Sciences Andrew Farach, Biology Madeline Gregorits, Biological Sci. Jessica Hall, Animal Science |
Patricia
Hansen, Biology Eric Hardter, Biochemistry Brett Hensley, Medical Technology Liang-I Kang, Biol Sci James Kelleher, Biochemistry |
Jamie
Kuczmarski, Physical Therapy Kellie Machlus, Biochemistry Vivek Patel, Biol. Sci. Kristen Reese, Biol. Sci Alfred Smith, Biochemistry |
| UDaily article
about Practice
Session at McKinly Lab March 7. |
UDaily
article about the trip. |
Photo Gallery of Trip |
|
Akt is Preferentially Expressed in PC-3 Cells Adhering to Bone Matrix Nicole
Barkley, Freddie Pruitt, *Kathleen Ignatoski, Bianca Graves, Meghna
Naik, Ulhas P. Naik, Carlton
R. Cooper University of
Delaware, Biological
Sciences, |
Prostate
cancer (PC) commonly metastasizes to the skeleton, where it becomes
highly resistant to chemo-therapeutics. The components of the bone
extracellular matrix microenvironment and other secreted factors have
been implicated in PC cell proliferation. The purpose of this project
was to determine
the effects of PC-3 cells adhering to bone substrates on Akt
activation.
PC-3 cells were plated on substrates composed of secreted factors from
osteoblastic-like cells (SaOS2), bone stromal-like cells (HS-5), as
well
as collagen type-1, a major component of the bone matrix, and
fibronectin.
Western analysis was performed to resolve the phosphorylated Akt. We
hypothesize
that prostate cancer cells grown on the substrate of secreted factors
from
osteoblastic (SaOS2) and bone stromal (HS-5) cells will preferentially
stimulate the activation of Akt. Also we hypothesize that PC-3 cells
grown
on type-1 collagen will preferentially stimulate Akt activation rather
than
grown on fibronectin. Western blot analysis of Akt activation showed
the
greatest increase in PC-3 cells on SaOS-2 secreted substrate compared
to
the control (plastic) and HS-5 conditioned media. In addition, we
present
data showing that Akt is activated preferentially on collagen-type 1
compared
to fibronectin. Lastly, we demonstrate that ErbB2 is preferentially
expressed
on PC-3 cells grown on soluble bone-marrow extracts compared to kidney
extracts.
These data suggest that secreted factors, including collagen type I,
predominately
from osteoblastic cells, enhance prostate cancer cell survival in the
bone
microenvironment and these factors may contribute to drug resistance. |
|
ASBMB Honorable
Mention
|
Hyperphosphorylation of tau induces local structural changes Agata
Bielska and Neal
J. Zondlo |
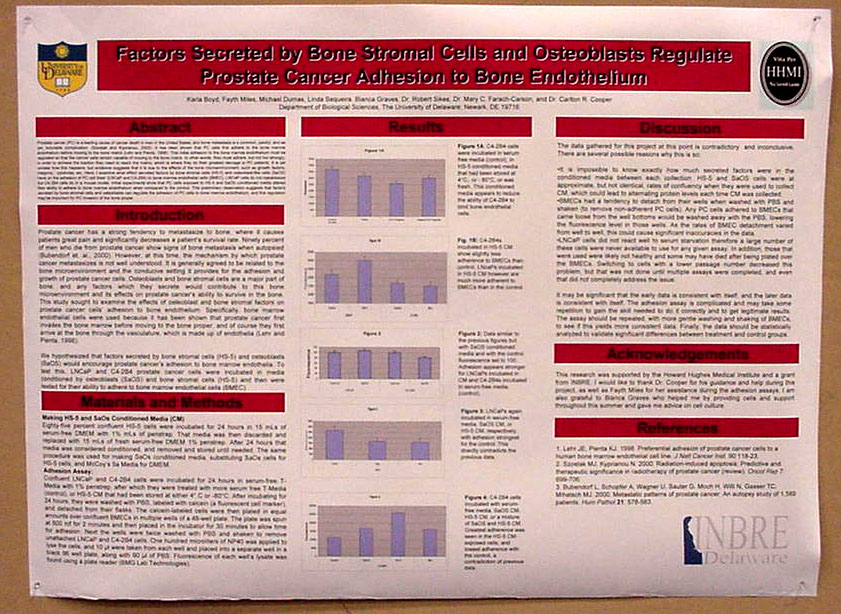 Factors Secreted by Bone Stromal Cells and Osteoblasts Regulate Prostate Cancer Adhesion to Bone Endothelium Karla Boyd, Fayth Miles, Michael Dumas, Linda Sequenia, Bianca Graves, Robert Sikes, Mary C. Farach-Carson, and Carlton Cooper Department
of Biological Sciences, The |
Prostate
cancer (PC) is a leading cause of cancer death in men in the
|
Biological
Sciences, University of Delaware, 248 Wolf Hall, Newark, Delaware,
19716 |
Interest
in modification of proteins by O-linked beta-N-acetylglucosamine
(O-GlcNAc) is growing rapidly due
to its continued implication in the regulation of cellular processes.
Since roles for O-GlcNAc in brain development have not yet been
described, we
characterized the expression of O-GlcNAc in the developing chick
midbrain
(optic tectum) to see if patterns existed in specific cell types, in
subcellular
processes like axons, or in specific proteins. Based on the recurrent
O-GlcNAcylation of highly phosphorylated proteins in other vertebrate
systems, neurofilament modification in neuronal axons was examined. Our
studies indicate that
a regulated developmental pattern of O-GlcNAcylation exists in the
developing chick brain. Immunohistochemical analyses in sections of
progressive stages of development suggest upregulation of O-GlcNAc in
the ependyma, tectobulbar neuron bodies, and radial glial processes,
but not in neurofilament (+)
axons. In contrast, O-GlcNAcylation of most axons occurred in cultures
made
from embryonic day 7 brain cells. Western blot analysis showed O-GlcNAc
modification of a few discrete proteins throughout development,
including
one of approximate weight of vimentin (52kDa), an intermediate filament
marker of radial glia in the chick brain. These results and
labeling
of radial glia in brain sections indicate that it is vimentin that is
modified
by O-GlcNAcylation. This potentially indicates an undescribed
developmental
role for O-GlcNAc modification that could include vimentin intermediate
filaments as well as neurofilaments. Funded by HHMI, Charles
Peter
White Fellowship, and UD Undergraduate Research Program. |
|
Madeline
Gregorits, Ronald R. Gomes, Jr. Department of Biological Sciences |
There
are few models available to study spontaneous osteoblastic prostate
cancer metastasis to bone. Current models require orthotopic injection
and more than six months to form metastases in bone, while intracardiac
injection, specifically with PC-3 cells, results only in osteoclastic
bone metastases. We have hypothesized that differences in bone turnover
between mice and humans is the main limitation for the spontaneous and
rapid colonization of marrow forming bone by osteoblastic prostate
cancer cells, as bone turnover rates in adult mice do not mimic the
higher, continuous turnover rates observed in humans. SCID/bg
mice were subjected to two and three week periods of tail suspension to
enhance
bone turnover, followed by intracardiac injection of C4-2 prostate
cancer
cells and a return to normal weight bearing activity. MicroCT analysis
revealed
a significant reduction in trabecular bone volume and an increased
porosity
in the femurs of tail-suspended mice relative to controls. After three
weeks
of tail suspension and six weeks post C4-2 cell injection, 100% of the
mice
demonstrated detectable serum PSA levels. Immunohistochemical staining
of
femurs for PSA revealed the localization of PSA positive, C4-2 cells in
marrow and trabecular spaces. These results suggest that this novel
model
combining tail suspension in an immune compromised mouse strain and
intracardiac
injection could allow for the direct testing of therapeutic agents that
interfere with the process of osteoblastic prostate cancer colonization
of
bone. Funding provided by the Howard Hughes Medical Institute and
University of Delaware Research Foundation. |
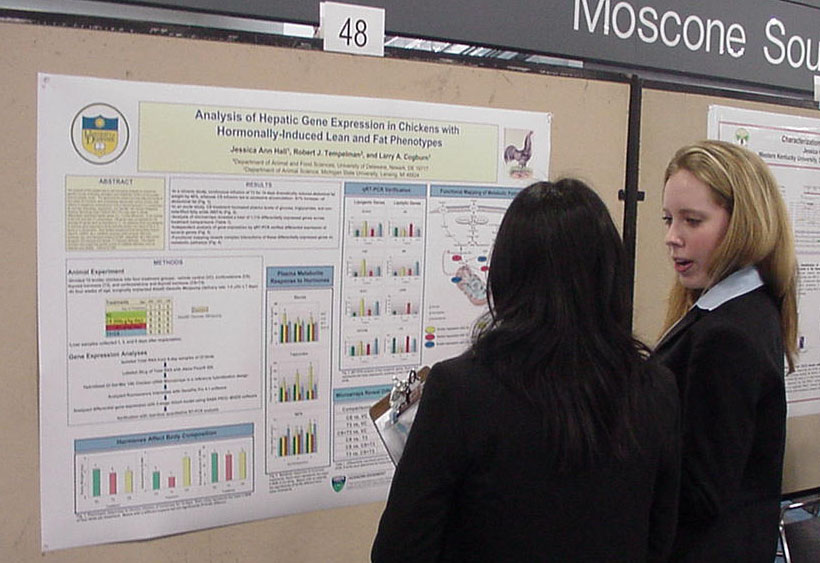 Analysis of hepatic gene expression in chickens with hormonally-induced lean and fat phenotypes Jessica Ann Hall1, Robert J. Templeman2, and Larry A. Cogburn1: 1Department of Animal and Food Science, University of Delaware, Newark, DE 19717, 2Department of Animal Science, Michigan State University, East Lansing, MI 48824-1225 |
The
purpose of this project was to use microarray analysis to unravel the
genetic circuits controlling
deposition and metabolism of fat in a hormonally-induced obesity
model.
The abdominal fat content and free fatty acid levels of four-week-old
chickens were dramatically altered after chronic infusion (two weeks)
of exogenous corticosterone (CS) or thyroid hormone (T3),
producing a fat and lean phenotype, respectively. Our Del-Mar 14K
Chicken Integrated Systems Microarray (Geo Platform GPL1731) was used
to identify differentially expressed hepatic genes (i.e., 1.4-fold
difference). In the contrast of fat
(CS) and lean (T3) phenotypes, 136 genes were up-regulated
by
CS, whereas 64 genes were up-regulated by T3. An
additional
study was designed to examine gene response to acute hormone infusion
(six
days) of CS or T3, alone or in combination. Both
studies
revealed several transcription factors (Spot14, CEBP2, etc.), metabolic
enzymes
(malic enzyme, fatty acid synthase, etc.) and transport proteins
(LFABP,
adipophilin, etc.) that control lipogenic (CS induced) and lipolytic (T3
induced) pathways. This project provides new insight into genetic
control
of metabolic disorders, such as diabetes and obesity. This work
was
supported by a USDA Training Grant (2004-38411-14734) and by a
USDA-IFAFS
Animal Genome Program (00-52100-9614). ASBMB Honorable
Mention
|
Biological Sciences, University of Delaware,
248 Wolf Hall, Newark, Delaware, 19716 |
Breast
cancer metastases to brain are often rapidly lethal. This project has
established the chick embryo as
a new model for studying extravasation of breast cancer to brain. Human
MDA-MB-231 and MDA-MB-435 breast cancer cell lines were infected with
retroviral
vectors expressing the lacZ marker gene and/or the green fluorescent
protein
(GFP) gene for identification of cells in vivo and in vitro.
Approximately
50,000 cells were injected into the extraembryonic vasculature of 5 day
embryos, followed by dissection of the brain, fixation and
beta-galactosidase
staining, and preparation of routine H&E paraffin sections less
than
2 weeks later. Cryosections were also prepared and subsequently
immunostained
for marker genes. Numerous lacZ(+) tumor cells were present on the
outside
of the brain after 10 days in the embryo, and cells were clearly
located
in brain tissue outside blood vessels, demonstrating their
extravasation.
GFP expressing tumor cells were recovered from dissociated brains and
subjected
to quantitation by flow cytometry and clonal formation analyses after
puromycin
drug selection. They were detectable by flow cytometry, but drug
selection
and clonal analysis was a more sensitive and accurate method of
quantitation
of tumor cell extravasation into brain. These experiments have
validated
the use of this new model for the study of breast cancer metastasis to
brain,
and now will allow molecular mechanisms of metastasis to be
investigated.
Furthermore, lacZ and GFP both were useful markers for localization and
quantitation of tumor cells. Supported by the Howard Hughes Medical
Institute,
the Charles Peter White Fund, and the University of Delaware
Undergraduate
Research Program. |
|
MAX
1 is a 20 amino acid beta-hairpin comprised of valine-lysine repeats
and a type II' turn sequence of -ValD-ProProThr-. This peptide
undergoes triggered folding from random coil to beta-hairpin
conformation. Subsequent self-assembly of the hairpins affords hydrogel
material which is currently being investigated for use in tissue
engineering. Circular dichroism is used extensively to assess the
secondary structure
of these peptides during folding and self-assembly. However, it is not
known definitively how the turn sequence contributes to the
spectroscopic data obtained. To elucidate this matter, a series of
6-residue turns (C-V-X-X-T-C) have been synthesized, and subjected to
circular dichroism, nuclear magnetic resonance, and infrared
spectroscopic methods. The contribution of the
turn region to the overall spectroscopic signal of the hairpin will be
discussed. Furthermore, all experiments were performed under both
oxidizing and reducing conditions, so as to further determine the
impact of a disulfide bond on these turns. This research was supported
by the HHMI Undergraduate Science Education Program. ASBMB Honorable
Mention
|
|
Brett
Hensley, Carrie Paquette-Straub, Department of
Medical Technology, |
Disintegrins,
low molecular weight proteins
isolated from snake venom, can interact with many cell types and
adhesion
molecules. One disintegrin, eristostatin (Er), has the ability to
inhibit
platelet aggregation and human and murine melanoma cell metastasis.
Recent studies
using wound healing assays suggest that Er may inhibit melanoma cell
motility.
No mechanism elucidating Er’s mode of inhibition has been discovered
thus far.
Using immunoprecipitaton with antiphosphotyrosine antibody, we
evaluated the
effect of Er (3000nM) on tyrosine phosphorylation of intracellular
proteins
within five human melanoma cell lines: C8161, M24met, 1205 LU, MV3, and
WM164.
We determined that Er notably changed the protein phosphorylation
within all
five cell lines. The 1205 LU cell line showed an increase in protein
phosphorylation from 100-140 kDa, 60-75 kDa, and 37-45 kDa when treated
with Er
compared to cells treated with water. M24met cells showed a similar
increase in
protein phosphorylation at 95-100 kDa and 40-45 kDa when treated with
Er. A
decrease in protein phosphorylation was found at 40-45 kDa for
Er-treated C8161
cells and at 95-100 kDa and 135-140 kDa for Er-treated MV3 cells.
Er-treated
WM164 cells showed a decrease in protein phosphorylation at 55-60 kDa
and
135-140 kDa as well as an increase at 125-130 kDa. A review of all
known
tyrosine phosphorylated proteins involved with cellular motility
provided many
possible candidates including Pten, EGFR, Fak, FGFR, PDGFR, Pyk2, Hck,
and
others. Using another approach, without immunoprecipitation, in order
to
examine the same intracellular phosphorylation yielded contrasting
results,
which will be examined and summarized. Further
experimentation evaluating this alternative method
is needed in
order to validate results. Research
supported
by NIH (CAO98056, MAM) and Charles Peter White Scholarship (BH). |
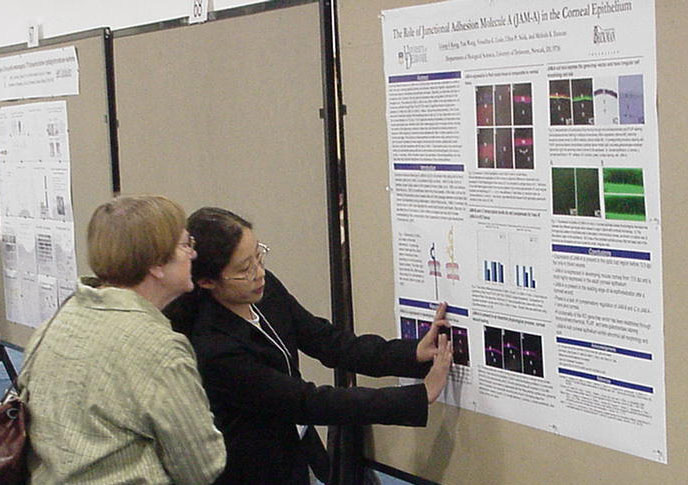 The Role of Junctional Adhesion Molecule A (JAM-A) in the Corneal Epithelium Liang-I Kang, Yan Wang, Vesselina Cooke, Ulhas P. Naik, and Melinda K. Duncan Department of Biological Sciences |
Junctional
Adhesion Molecule-A (JAM-A) is a 32 kDa protein that has been
implicated in a variety of roles in the body, including platelet
activation and adhesion, leukocyte migration, angiogenesis, and the
structural integrity of endothelial and epithelial cell layers.
Recently, our laboratory has begun to characterize JAM-A function in
the eye since its expression was upregulated in the lens of
Pax6 transgenic mice. The presence of JAM-A, JAM-B, and JAM-C
mRNA
in the adult wildtype lens and cornea was confirmed through Real Time
RT-PCR,
with no significant level of compensatory expression of JAM-B and JAM-C
in JAM-A -/- tissue. Using immunohistochemistry, JAM-A protein
was
found in the blood vessels of the developing eye as early as 12.5 dpc
(days
post coitum) and in the corneal epithelium by 13.5 dpc. PLAP
(placental
alkaline phosphatase) and beta-galactosidase reporter gene activity was
detected in the JAM-A heterozygous and homozygous cornea, indicating
the
insertion of the gene-trap construct in these mice, and
immunohistochemistry
showing the absence of JAM-A staining in knockout cornea validated both
JAM-A antibody specificity and the knockout genotype. We
hypothesize
that JAM-A has a role in the maintenance of the cornea and future
functionality
studies will include corneal wound healing and corneal permeability
assays.
This project has been funded by NIH and the Arnold and Mabel Beckman
Foundation. ASBMB Honorable
Mention
|
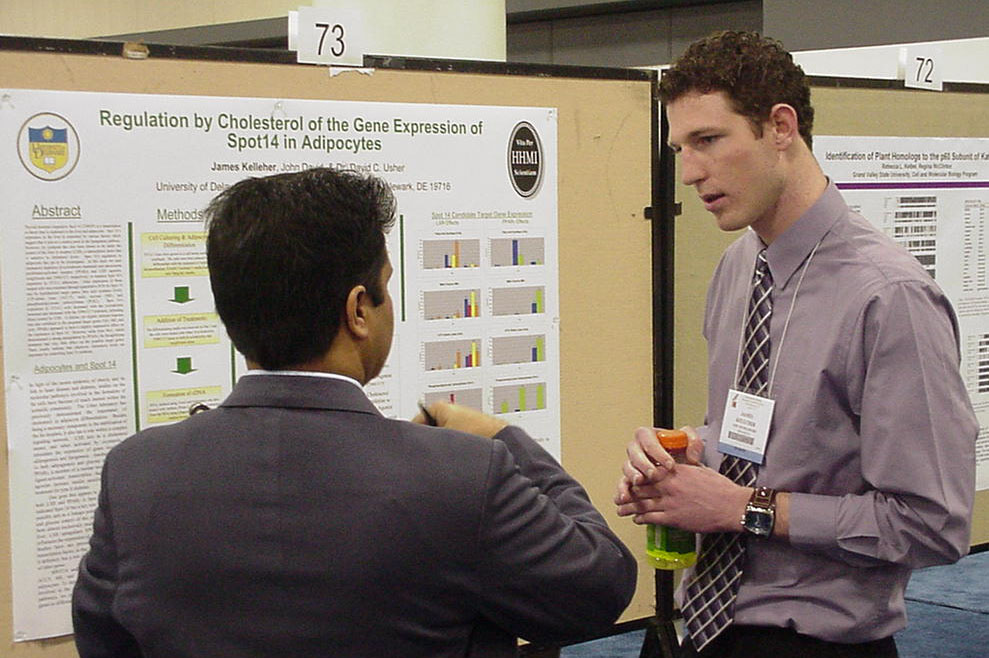 Regulation by Cholesterol of the Gene Expression of Spot 14 in Adipocytes James Kelleher, John David, and David Usher Department of Biological Sciences, University of Delaware |
Thyroid
hormone responsive Spot 14 (THRSP) is a transcription co-factor that is
expressed in the liver and adipocytes. Spot 14’s expression in
the liver is controlled by various factors which suggest that it acts
as a control point in the lipogenesis pathway. However, its synthesis
has also been
shown to be under the control of the liver X receptor (LXR), a
transcription factor that is sensitive to cholesterol levels.
Spot 14’s regulation in adipocytes has yet to be investigated. In
this study we used cholesterol depletion (β-cyclodextrin treatment) and
peroxisome proliferator-activated receptor (PPARγ) and LXR agonists,
rosiglitizone and T0901317, respectively, to examine Spot 14’s
regulation in 3T3-L1 adipocytes. Gene expression of these treated
cells was examined through quantitative PCR for Spot 14 and its
hypothesized target genes, fatty acid synthase (FAS), ATP-citrate lyase
(ACLY), malic enzyme (ME), and phosphoenolpyruvate carboxykinase
(PCK1). Spot 14’s expression in 3T3-L1 cells decreased with the
cyclodextrin treatment and increased with the T0901317 treatment,
indicating direct control by LXR. T0901317 also induced
upregulation in Spot 14 target genes FAS, ME, and
ACLY, but the cyclodextrin treatment had little to no effect on these
genes.
Rosiglitizone treatment also had very little effect on Spot 14 or its
target
genes. These results indicate that adipocyte cholesterol levels
are
important for controlling Spot 14 synthesis. This research was funded
in
part by the Howard Hughes Medical Institute Undergraduate Science
Education
program. |
|
Lack of association between body mass index and acute hypertonic saline induced increases in blood pressure J.M.
Kuczmarski, E.P. Delaney, M.M. Wenner, A.V. Prettyman, M.E.
Stillabower, and W.B. Farquhar Department of
Health, Nutrition, and Exercise Sciences |
Salt
sensitivity of blood pressure (BP) is thought to be greater in obese
subjects compared to normal weight individuals. The mechanisms
underlying sodium-induced increases in BP have not been fully
elucidated. We hypothesized that body mass index (BMI) would be
associated with sodium-induced increases in BP. We
retrospectively examined data from 44 healthy subjects (mean ±
sem: 251 yrs) that completed a standardized 60-minute intravenous
infusion (0.15 ml/kg/min) of 3% sodium chloride (hypertonic saline
infusion: HSI). The HSI protocol is a robust sodium and volume
stimulus, and allows acute BP responses to be quantified. Blood
pressure was assessed on a beat-to-beat basis non-invasively with a
Finometer. In this cohort, BMI ranged from 19-35 kg/m2
(25±1 kg/m2). Serum
sodium increased pre- to post-infusion (135±0.6 vs.
141±0.4 mmol/L; p < 0.01) and hematocrit declined
(39±0.7 vs. 36±0.6 %; p < 0.01) during the 60-minute
HSI. Baseline mean BP was 80±2 and this increased to
90±2 mmHg (p < 0.01) at the end of the infusion. BMI
did not correlate with the increase in systolic BP (r=0.04,
r2=0.002, p=0.78), diastolic BP (r=0.11, r2=0.013, p=0.47), or mean BP
(r=0.03, r2=0.001, p=0.87). Also, no significant correlation was
found between skinfold-derived percent body fat and increases in mean,
diastolic,
or systolic BP. In conclusion, within a range of 19-35
kg/m2,
there was no association between BMI and the hypertonic saline-induced
increase
in BP. Supported by NIH grant R15 HL74851 and The University of
Delaware
Undergraduate Research Office |
Department of
Chemistry and Biochemistry |
Although
much is known about the structure and corresponding function of soluble
proteins, the folding mechanisms
and pathways of G protein-coupled receptors (GPCRs) are poorly
understood. Our goal is to understand the specific transmembrane
interactions that
stabilize integral membrane proteins, and to develop a model for GPCR
folding.
In this investigation, the Adenosine 2A Receptor (A2AR)
was studied as a model GPCR. Like all GPCRs, A2AR
contains seven membrane-spanning alpha-helical domains. Recombinant DNA
methods
were used to create a mini-gene of the A2A receptor that
contains
trans-membrane helices two and three, and another containing five and
six.
Using the pET31b(+) system, fusion constructs of these peptides have
been
sufficiently over-expressed in E. coli, and purified by Ni-Chelate
chromatography. Currently, studies are underway to characterize
the structure of the two-helix fragments, and measure its interactions
with other trans-membrane helices from A2AR. Ultimately, we
are using these and similar constructs to learn about the assembly
pathway and structure of A2AR. This project was funded
by HHMI and the NIH. |
Biological
Sciences, University of Delaware, 248 Wolf Hall, Newark, Delaware,
19716 |
Methods
generally in use to measure tumor cell movements lack sufficiency in
numbers of cells analyzed, precision of measurements, or temporal and
spatial resolution. Here, the study of glioma cell motility was
greatly enhanced using a fully automated time-lapse microscopy system
capable of collecting and analyzing motility data at
closely spaced time points (5 min), over long periods (24 hrs), and
under
several different experimental conditions in parallel. This system was
designed to be significantly more versatile and less costly than
commercial
systems and collected data under phase contrast and widefield
fluorescent
illumination concurrently. Human and rat glioma cell lines were
plated
under a variety of conditions and subjected to time-lapse microscopy,
cell
tracking, and quantitative analysis of velocity, total accumulated
distance, and directionality for individual cells or for averaged cell
populations. Quantitation of glioma —scratch“ assays revealed changes
in motility parameters after 1) anti-adhesion molecule
antibody-treatment, 2) adhesion molecule-transfection, or 3)
antisense-adhesion molecule viral vector infection. Fluorescently
labeled glioma cells were tracked while migrating on top of cell
monolayers
that expressed ectopic adhesion molecules, and this resulted in
significantly
reduced glioma migration velocities. Our methods of analysis
revealed
changes in glioma cell motility after experimental treatments that
would
not be discernable by other common methods. Supported by the HHMI
Undergraduate
Research Program, the McNair Scholars Program, and the U.D.
Undergraduate
Research Program. |
Kristen L. Reese and Patricia A. Martin-DeLeon, Department of Biological Sciences ASBMB Honorable Mention |
Fertilization
in mammals requires the successful completion of cumulus cell
dispersion, zona pellucida binding, and perivitelline space
penetration. The digestion of hyaluronan, which is abundant in
the cumulus cell matrix and zona pellucida, is essential for permeating
the vestment of the oocyte and is performed in the mouse by a family
of enzymes termed the reproductive hyaluronidases. HYAL3/Hyal3,
located
on human chromosome 3p21/mouse 9F1 and previously thought to be a
somatic
hyaluronidase, shares great similarity to identified reproductive
hyaluronidases,
yet there have been no studies to document a role in
fertilization. Recently it has been shown that sperm lacking
functional Spam1 are able to penetrate an oocyte, indicating related
hyaluronidases can functionally contribute to fertilization.
Interestingly, humans lack a functional reproductive hyaluronidase
other than SPAM1 on the 7q31 chromosome. Hyal3 has the highest
amino acid identity (80%) to its human homolog and shares high
testicular expression and identity to SPAM1. Therefore, it is
particularly important to investigate the potential role in the
fertilization process of humans in
addition to or in the absence of a functional SPAM1 protein.
Here, we
demonstrate that in addition to its testicular expression, Hyal3 is
also present
on the head of sexually mature sperm and is involved in acrosomal
exocytosis,
an important step in fertilization. Hyal3 is also present in
extra-testicular
tissues such as the epididymis and uterine luminal fluid of females in
estrus.
Based on the tissue expression patterns of Hyal3, its functional domain
similarities,
and involvement in acrosomal exocytosis, a redefinition of this protein
is
in order. Further investigation will be required for
reclassification
to extend to the human model. This research is funded by the
Howard
Hughes Medical Institute and NIH grant #R01 HD38273. |
|
Protein-protein,
protein-RNA, and protein-DNA interactions are broadly mediated through
the guanidinium functionality of arginine residues. However,
specific recognition
by arginine is limited, because the guanidinium functionality is
attached
to a linear alkyl group. To achieve specific molecular
recognition,
arginine mimetics are used, which place functional groups adjacent to a
guanidinium. In order to specifically target arginine-mediated
recognition,
we developed convenient syntheses of alpha- guanidino acids, in which
the
amine of an amino acid is converted into a guanidinium. The
alpha-substituted
guanidiniums of guanidino acids and the side chain of the amino acid
work
synergistically toward molecular recognition with greater affinity for
the
target site. We have designed arginine mimetics for specific and
high
affinity molecular recognition by coupling protected guanidino acids to
alcohol and amine nucleophiles. Protected guanidino acids of Gly, Phe,
Val,
and Leu were readily synthesized from methyl esters of alpha-amino
acids
by guanylation of the amine with bis-boc-thiourea and Mukaiyama’s
reagent.
Protected guanidino acids, with a free carboxylic acid for coupling to
nucleophiles,
were generated by saponification of the methyl ester using LiOH.
Arginine
mimetics were synthesized by coupling protected guanidino acids to
hydroxyl
and amino groups to generate complex alpha-substituted
guanidiniums.
Molecules containing alpha-guanidino acids were applied to specific
protein
and RNA recognition. This work supported in part by the National
Institute
of Health and the Howard Hughes Medical Institute Undergraduate Science
Education
Program. |
The trip to the Experimental Biology
Meetings in San Diego was organized by the University of Delaware
HHMI Undergraduate Science Education Program with additional support
from travel grants from the American
Society for Biochemistry and Molecular Biology, the Beckman
Scholars Program, and the University of Delaware's Women
Scholars Program. The HHMI Program,
the Beckman
Scholars Program, Charles Peter White Fellowships, the Biomedical Research
Infrastructure Network (BRIN) Program, a USDA Grant, and the Undergraduate Research Program
supported research by the students.
Trip Photos. Click on any image for a full-sized picture.
 UD alums Jennifer Paulson '99, Cory Ocasio '00, and John Dueber '99 all BS Biochemistry. |
San Francisco from the Golden Gate Bridge. |
 East Coast Tourists at Golden Gate with San Francisco in the back ground. |
|
Alcatraz |
 Walking tour of Chinatown in the rain. |
 Ghirardelli
Chocolate Factory in the rain. Ghirardelli
Chocolate Factory in the rain. |
 Blue and Gold Line Ships in San Franisico |
 Liang Kang, Aggie Bielska, and Kristen Reese talking to 1992 Nobelist Edmond Fischer who discovered protein phosphorylation. |
 Aggie Bielska, Jess Hall, and Al Smith with 1959 Nobelist Arthur Kornberg discoverer of DNA polymerase. |
Aggie Bielska, Jess Hall, and Al Smith with 1959 Nobelist Arthur Kornberg discoverer of DNA polymerase. |
 UD students at the ASBMB Undergraduate Poster Competition. |
 UD awardees (1,3,4,5 front row, 3 in back row) with Tom Cech, President of HHMI |
 UD Students who received Honorable Mentions in the ASBMB Poster with Tom Cech and others. |
 Colorful seafood at Fisherman's Wharf. |
 Front Range of the Rocky Mountains from 32,000 feet. |
 Aggie, Brett, Dr. Laverty, and Eric at Thai restaurant |
 Before or after the swim? |
 Yes, They actually did go swimming in the Pacific in March! |
|
 Hal White with Marilee Benore-Parsons (UD'86), a former graduate student of his. |
At Lefty O'Doules in the evening. |
 Dinner out with Dr. Usher. |
 Alumni
Dinner Alumni
Dinner |
. Ron Gomes talking with Dave Usher at the UD alumni dinner. |
 Alumni Dinner |
 Alumni Dinner |
 Alumni Dinner |
 UD Students talking to 1992 Nobelist Edmond Fischer. |
 Waiting to depart PHL. |
 Waiting to Depart SFO |
 SFO
from the air. SFO
from the air. |