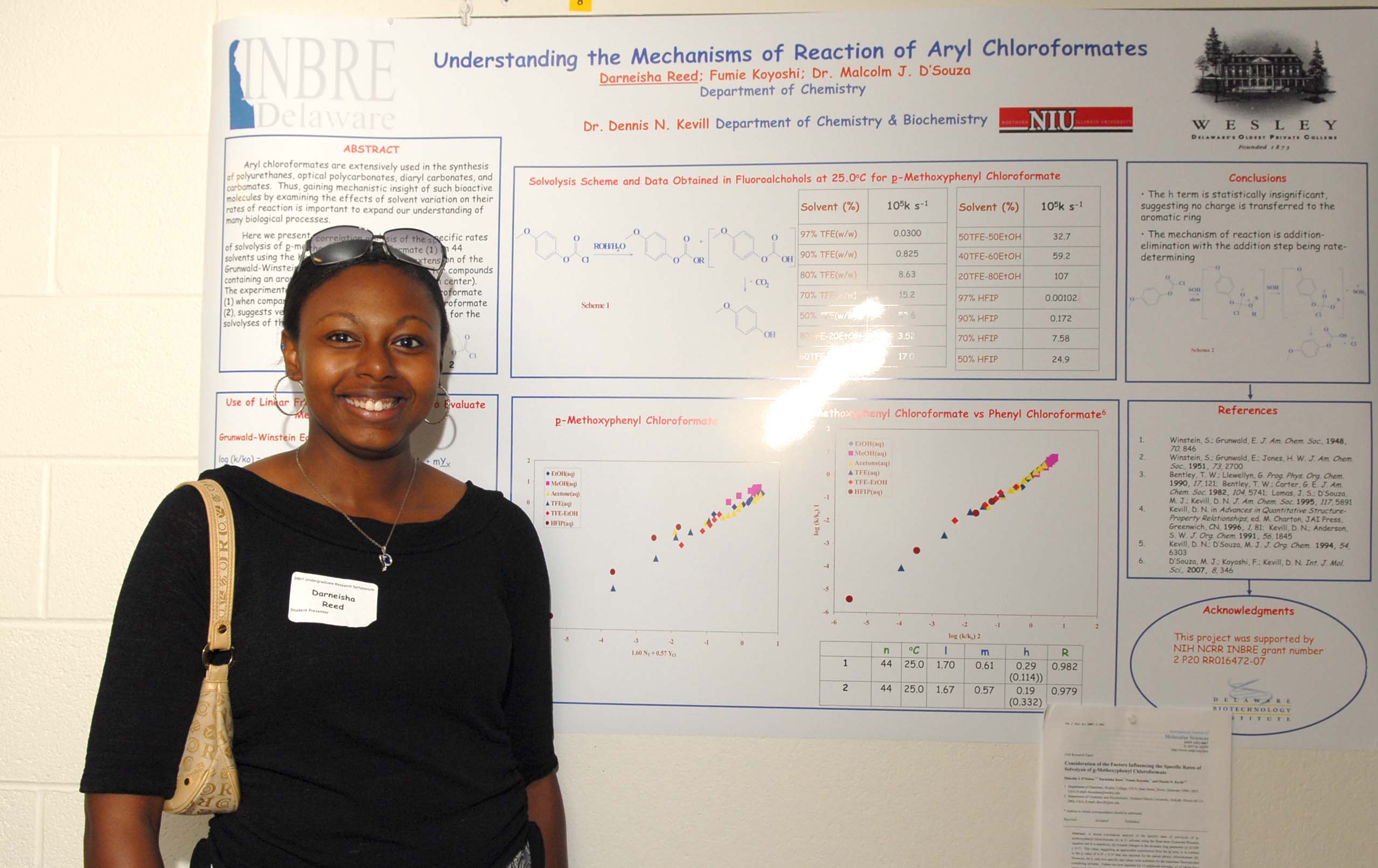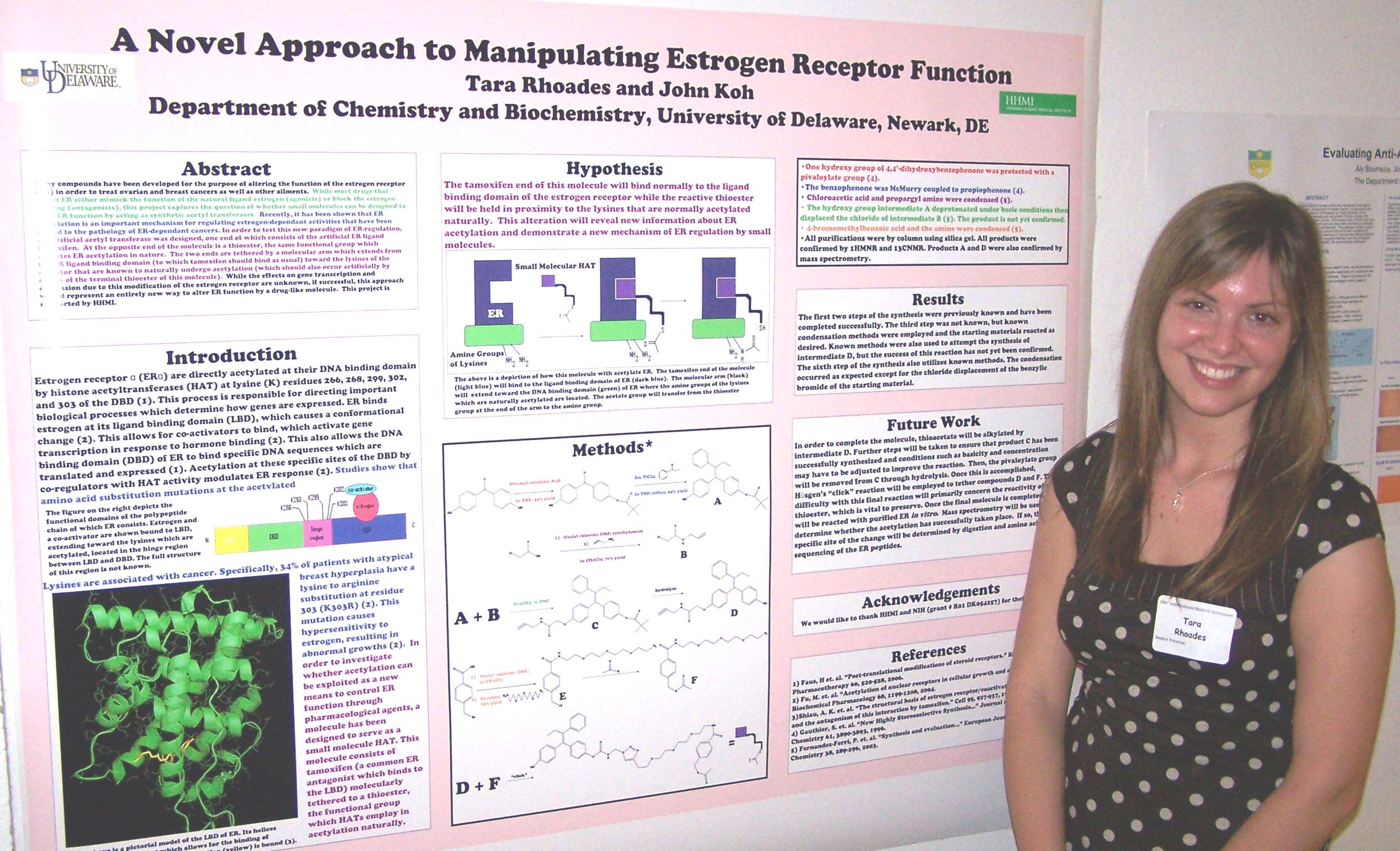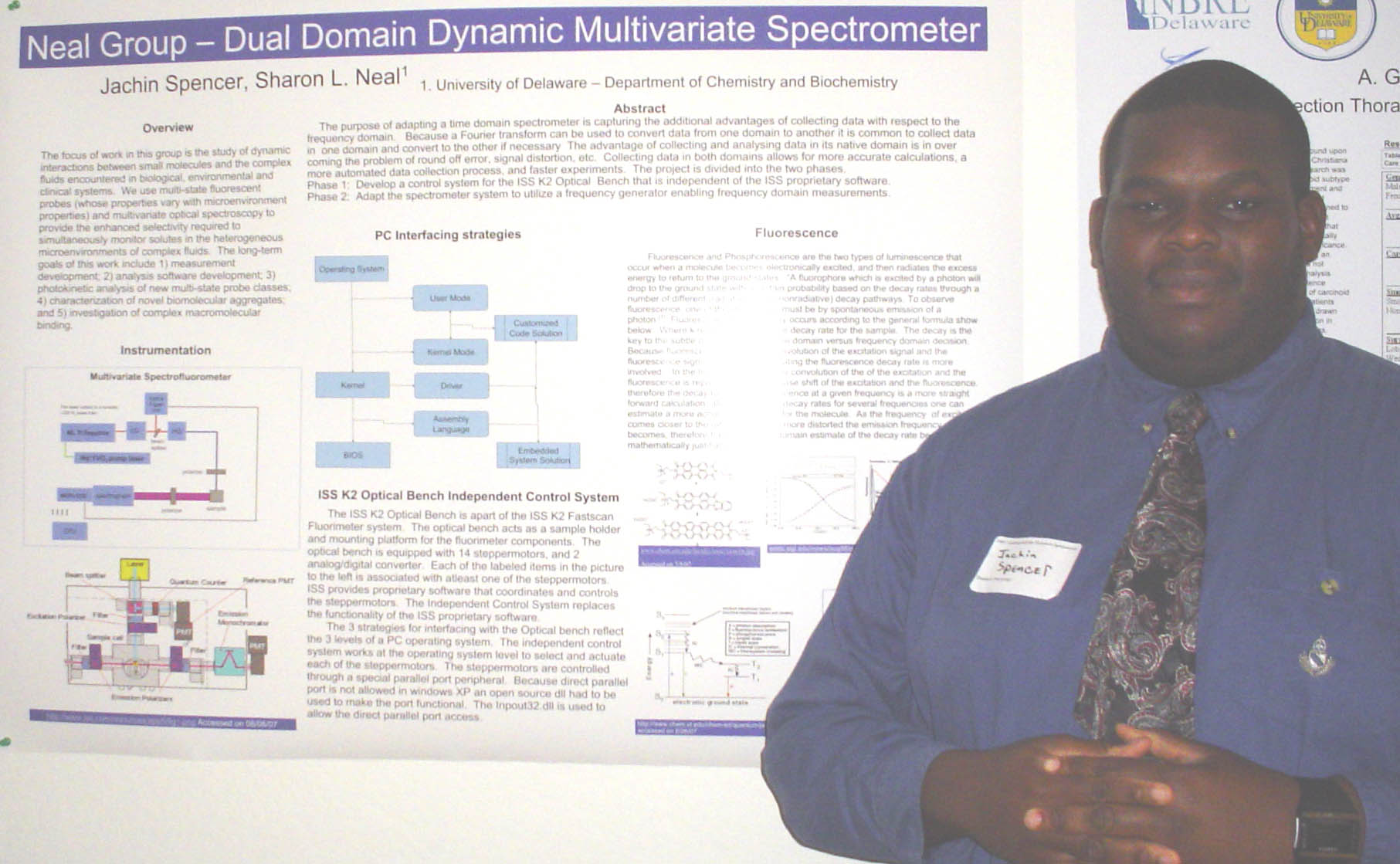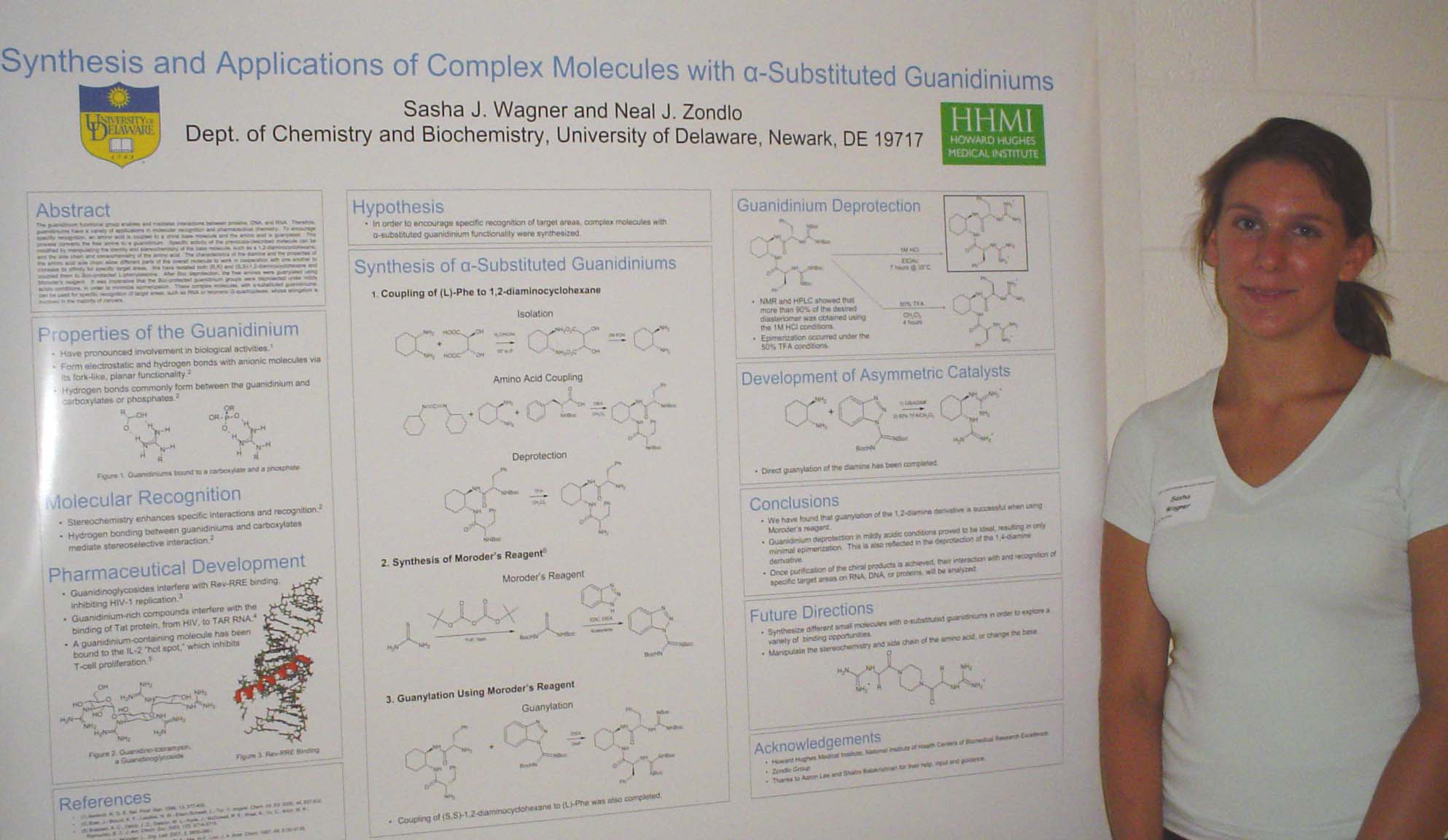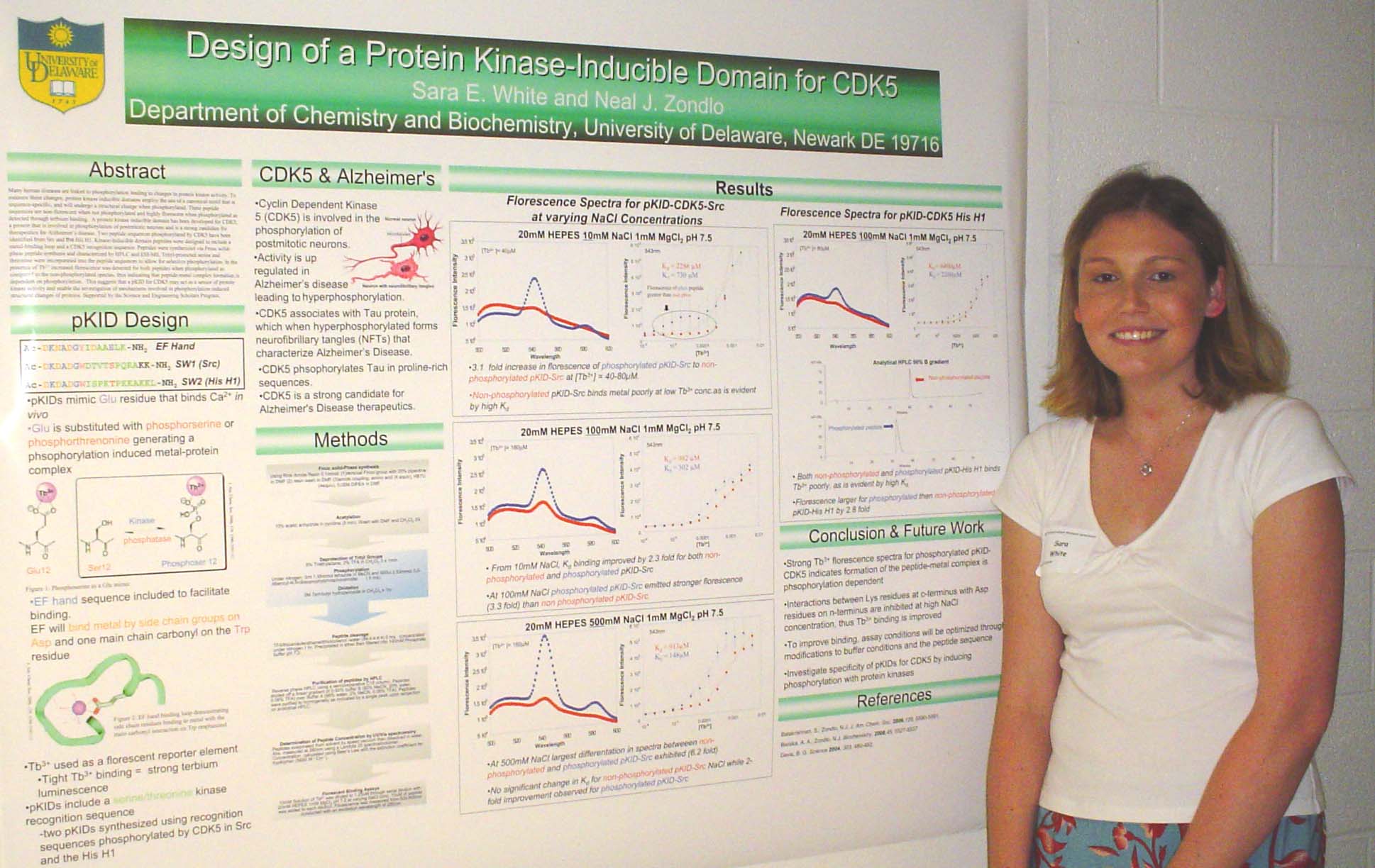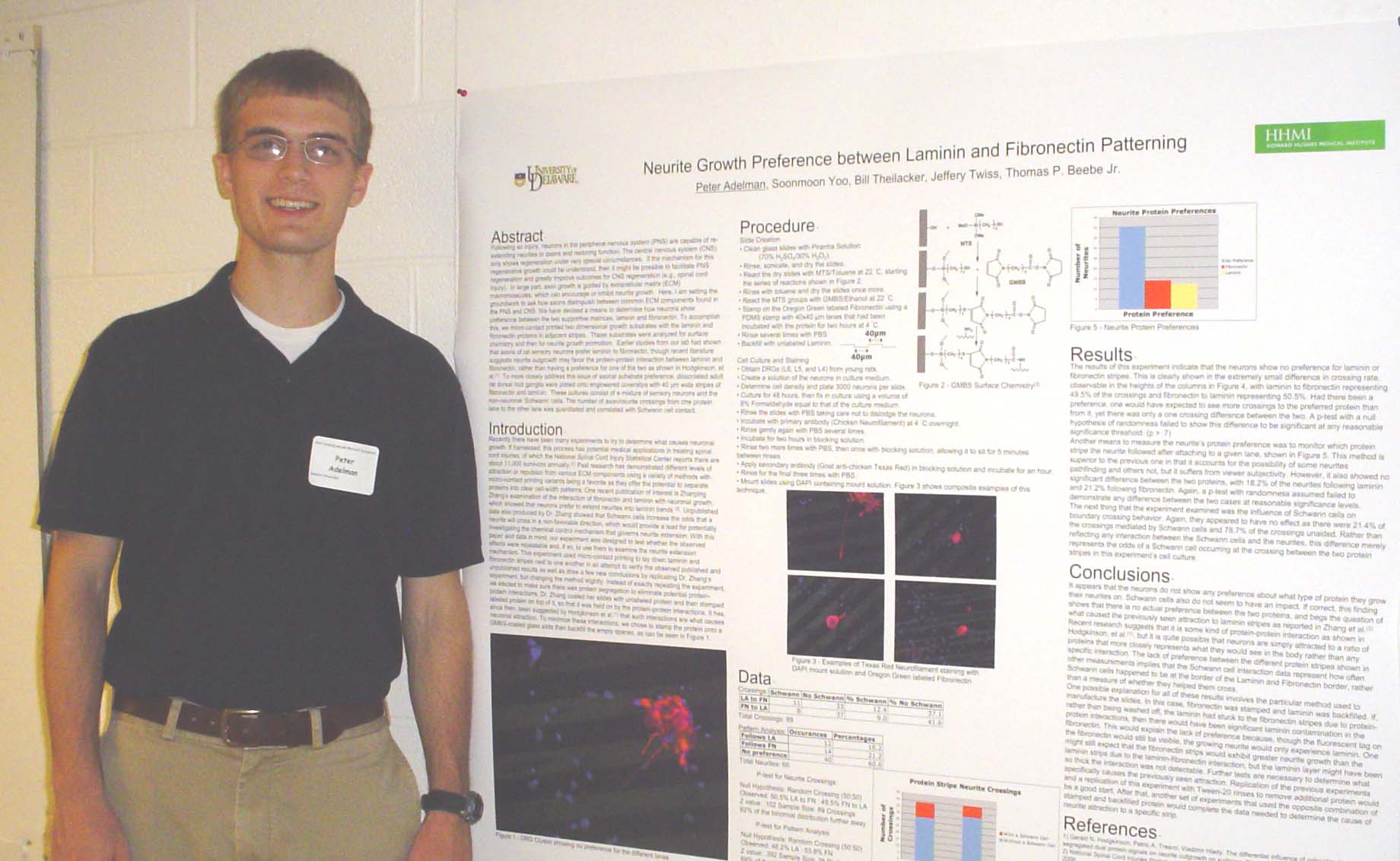
Neurite Growth Preference between Laminin and Fibronectin Patterning
Peter Adelman, Soonmoon Yoo, Bill Theilacker, Jeffery Twiss, and Thomas P. Beebe
Department of Chemistry and Biochemistry and the
Following an injury,
neurons in the peripheral nervous
system (PNS) are capable of re-extending neurites or axons and
restoring
function. The central nervous system (CNS) only shows regeneration
under very
special circumstances. If the mechanism
for this regenerative growth could be understood, then it might be
possible to
facilitate PNS regeneration and greatly improve outcomes for CNS
regeneration
(e.g., spinal cord injury). In large
part, axon growth is guided by extracellular matrix (ECM)
macromolecules, which
can encourage or inhibit neurite growth. Here,
I am setting the groundwork to ask how axons
distinguish between
common ECM components found in the PNS and CNS. We have devised a means
to
determine how neurons show preference between the two supportive
matrices,
laminin and fibronectin. To accomplish this, we micro-contact printed
two
dimensional growth substrates with the laminin and fibronectin proteins
in adjacent
stripes. These substrates were analyzed
for surface chemistry and then for neurite growth promotion. Earlier studies from our lab had shown that
axons of rat sensory neurons prefer laminin to fibronectin, though
recent
literature suggests neurite outgrowth may favor the protein-protein
interaction
between laminin and fibronectin, rather than having a preference for
one of the
two (Hodgkinson, et al., 2007). To more
closely address this issue of axonal substrate preference, dissociated
adult
rat dorsal root ganglia were plated onto engineered coverslips with 40
µm wide
stripes of fibronectin and laminin. These
cultures consist of a mixture of sensory neurons and
the
non-neuronal 'Schwann' cells. The number of axon/neurite crossing from
one
substrate to the other was quantitated and correlated with Schwann cell
contact. Supported by HHMI.