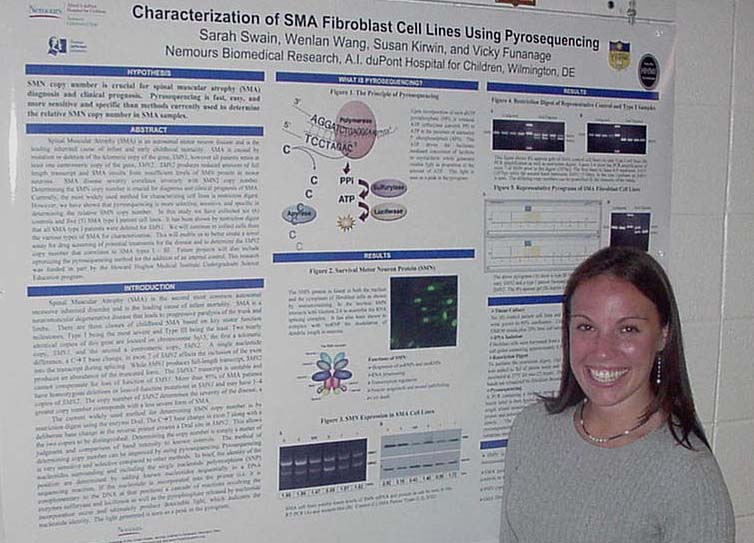
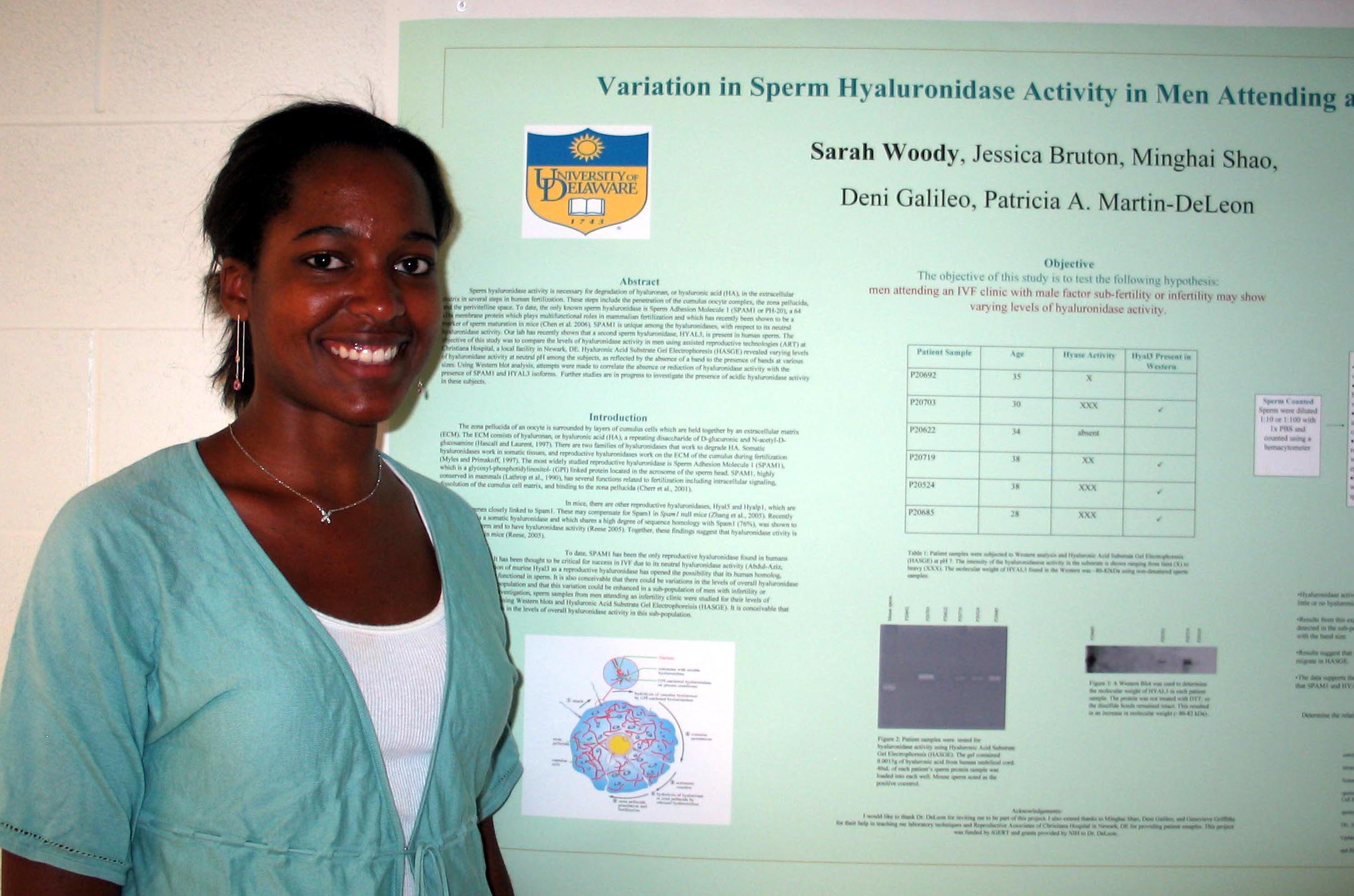
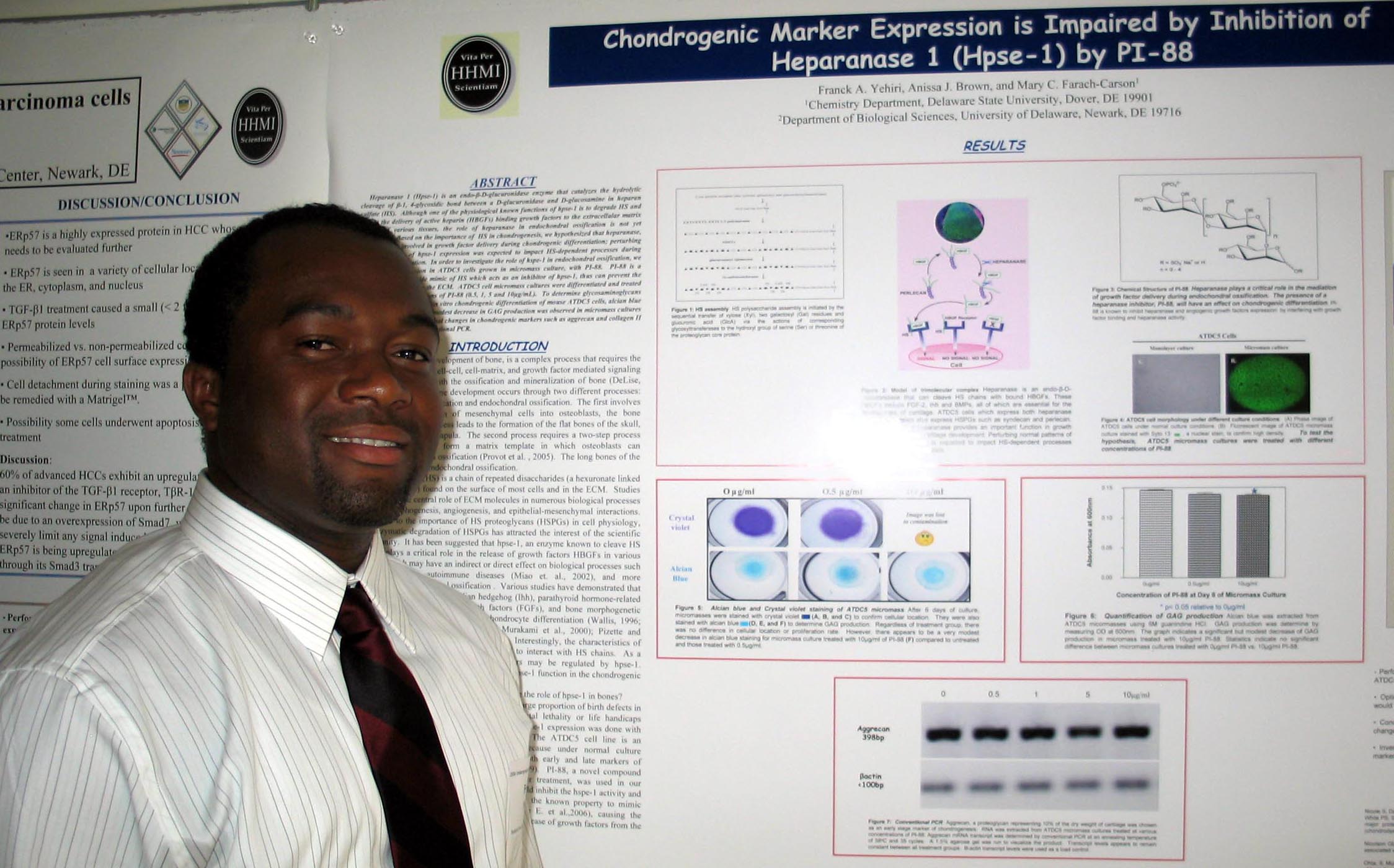
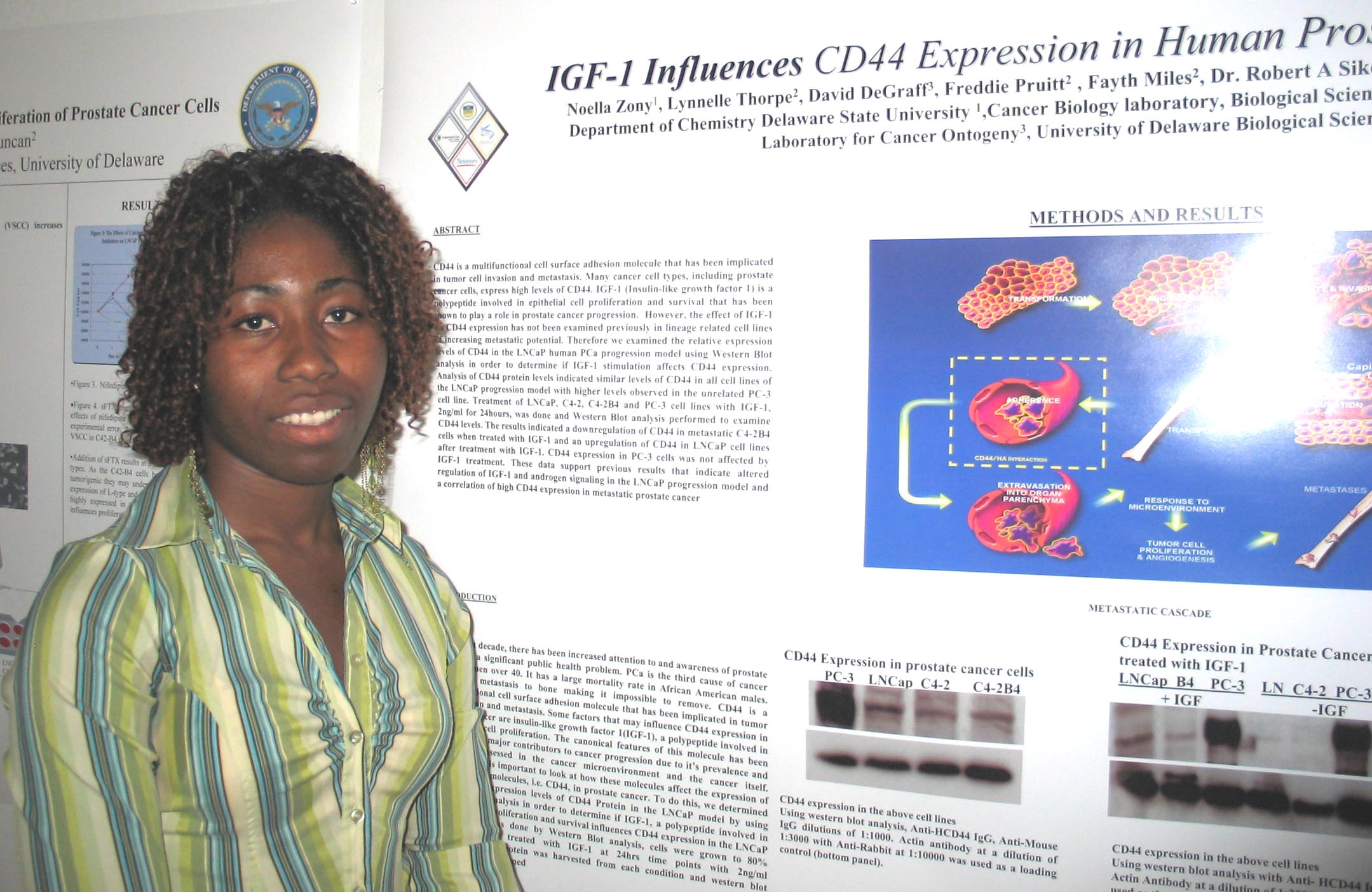
 Cholesterol Depletion in Adipocytes and its Affects on Lipoproteins E and C1 Marysol D. Lavander, Sara Sterling, and David Usher Department of Biological Sciences Apolipoprotein (apo) C1,
a 6.6-kD
protein, is a protein constituent of triglyceride (TG) -rich
chylomicrons, very
low density lipoproteins (VLDL) and high density lipoproteins (HDL).
The human APOC1 gene is located 5 kb downstream of
the human APOE gene on chromosome 19
in the same transcriptional orientation. ApoE mediates the uptake of
triglyceride-rich lipoproteins (TRL) by hepatic receptors, where as
apoC-I has
an inhibitory effect. Both these proteins appear to be involved in
cholesterol
efflux in adipocytes. In order to find
how this process is controlled, the expressions of APOE
and APOC1 were
tested in 3T3-L1 mouse adipocytes treated with β-cyclodextrin,
triiodothyronine
(T3) and a combination of β-cyclodextrin and T3. The results showed
that
β-cyclodextrin decreased expression of the two genes 7-10 fold,
T3
decreased expression 2-4-fold and the combination of β-cyclodextrin
and T3 inhibited expression 9-14 fold. The results suggest
that cholesterol depletion or stimulation of carbohydrate metabolism
causes
cells to attempt to increase the cholesterol concentration within the
cell.
This may be due to the formation of lipid droplets which require a
boundary of
cholesterol, phospholipids and protein. Sponsored by INBRE.
|