Metal Sulfide Clusters In Collaboration with Dr. Luther’s research group in the College of Marine Studies here at the University of Delaware we are working to characterize the chemical and physical properties of metal sulfide complexes such as CdS. Specifically, we are interested in understanding the process by which dissolved species condense to form solid products and to gain insight into the mechanism of nucleation and particle growth of metal sulfides. Our FT-ICR facility provides us with the tools needed to probe these complex systems. In past studies we have successfully analyzed similar systems using laser desorption (LD) ionization techniques and we plan to expand our analytical toolbox by using both matrix assisted laser desorption ionization (MALDI) and electrospray ionization (ESI) techniques. The high resolving power and mass accuracy capabilities of our instruments allow us to obtain critical information from the complex isotopic distributions commonly observed in mass spectra of metal clusters. |
|
Fragmentation Mechanisms of Oxidized Peptides FT-ICR mass spectrometry is a powerful tool for probing gas phase fragmentation mechanisms. Currently, one of our projects involves examining new dissociation pathways resulting from the oxidation of peptides. Previous studies have determined which amino acids are susceptible to oxidation but little is known about the effect this has on fragmentation within the mass spectrometer. The system we have chosen to investigate is angiotensin II which typically fragments to selectively form the y7 ion. Upon oxidation of Tyr the selective formation of the b4 ion is observed and upon oxidation of His the b5 selective dissociation channel is induced. In collaboration with Dr. Julia Laskin at Pacific Northwest National Laboratory, we were able to determine the energetics of these gas phase reactions using energy resolved surface induced dissociation (SID). These results and the use of molecular mechanics calculations have allowed us to gain insight into the mechanistic characteristics of these fragmentation channels. |
|
| Binding Energies of Metalloporphyrins |
|
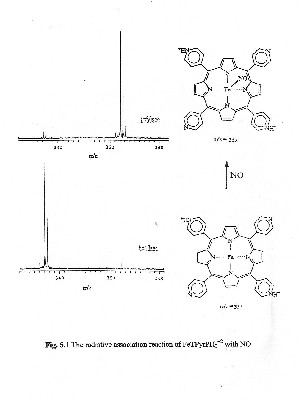 |
The ability of our instruments to act as a gas phase reaction chamber allows us to monitor chemical reactions over time providing us with the ability to understand the fundamental thermochemical properties of the reaction. In particular we are interested in understanding the bonding characteristics of matalloporphyrins bound to ligands such as NO, NO2, CO, O2 and O. We are able to determine bond strengths between metalloporphyrins and small molecules by measuring rate constants and equilibrium constants for gas phase thermal association and dissociation reactions. SID experiments are then used to probe the energetics and dynamics of the reactions. |
| Gas Phase Vibrational Spectroscopy using IRMPD FT-ICR MS | |
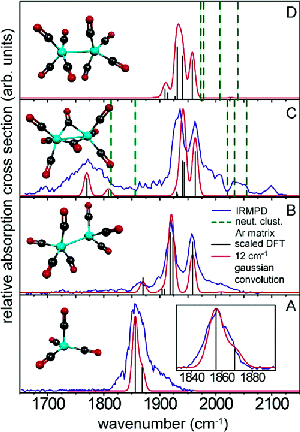 |
In addition, we are interested in studying the bonding modes of the various metalloporphyrin complexes by using IRMPD to measure vibrational frequencies. In the past we have collaborated with the FOM Institute for Plasma Physics to study similar systems where we reported the first gas-phase IR spectra of anionic iron carbonyl clusters. |