
|
Department of Chemistry and Biochemistry University of Delaware Newark, DE 19716 Phone: 302-831-2561 Fax: 302-831-6335 Lab: 302-831-2625 Email: kbooksh@udel.edu
|






|
Research Areas Broadly speaking, we are working in four related applications biomedical process monitoring, environmental process monitoring, industrial process monitoring, and chemometrics. |
|
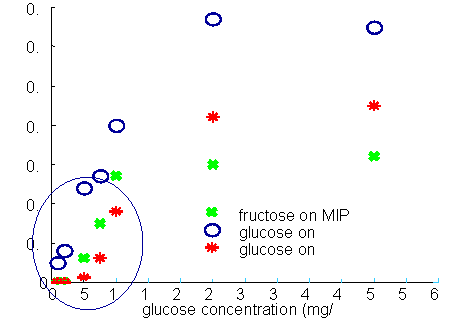
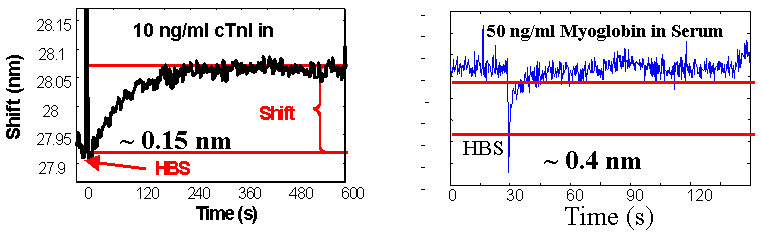
Biomedical Process Monitoring We have designed a fiber optic, surface plasmon resonance (SPR) sensor capable of in-situ detection on ng/ml protein levels in biological fluids. The key to this research is the development of organic coatings for the sensors that enable antibody attachment while still being resistant to non-specific binding from off-target proteins. This sensor is being applied to protein biomarkers for heart attacks, strokes, and wound healing. We are developing nanoparticle embedded, molecular imprinted polymers (MIP) for detection of small molecules by SPR spectroscopy. MIPs increase the sensitivity and selectivity of the surface coatings while employing nanopartical further amplifies the change in the SPR signal. With nanoparticle embedded SPR sensors we can selectively detect 0.1 mg/ml sucrose in urine and <40 ppm ammonia in vapor and aqueous phase samples. |
|
Calibration of glucose SPR-MIP sensor in water
|
|
1. “Quantitative Measurement of Cardiac Markers in Undiluted Serum” J.F. Masson, T.M. Battaglia, P. Khairallah, S. Beaudoin, K.S. Booksh, Analytical Chemistry 79 (2) 612-619, (2007). 2. “Reduction of Non-Specific Protein Binding on Surface Plasmon Resonance Biosensors” J.-F. Masson, T.M. Battaglia, J. Cramer, M. Sierks, S. Beaudoin, K.S. Booksh, Analytical and Bioanalytical Chemistry 386 (7-8) 1951-1959 (2006). 3. Molecularly imprinted polymer based surface Plasmon resonance sensing for glucose detection in human urine,” S.N. Banerji, W. Peng, Y.-C. Kim, K.S. Booksh, in SPIE Proceedings 6380 (2006). 4. “Quantitation of Cytokines in Highly Fouling Media using Surface Plasmon Resonance”, T.M. Battaglia, J.-F. Masson, S. Beaudoin, M. Sierks, K.S. Booksh, Analytical Chemistry, 77 7016-7023 (2005). |
|
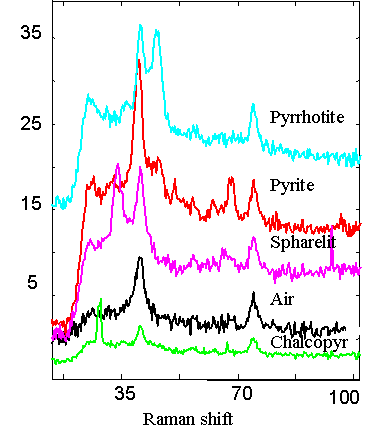
Environmental Process Monitoring We are developing field portable Excitation-Emission Matrix (EEM) fluorescence, Raman and Surface Plasmon Resonance spectrometers for detection of trace organics in natural water and air samples. These systems are designed for rapid screening and characterization of samples to achieve spatial/temporal maps of changes in target analyte concentration. We are also making the sensors fiber optic based for remote and dispersed analyses. The EEM fluorometeris being applied to monitor natively fluorescent analytes such as polycyclic aromatic hydrocarbons (PAHs) that are indicative of fuel spills. performing multi-way chemometric analysis methods enables quantitative analysis in the presence of unknown, uncalibrated interferants. We are also combining the EEM with photocatalytic degradation with EEM fluorescence to increase selectivity and expand the types of analytes that we can detect. SPR spectroscopy, with novel polymer coatings, is being developed to detect ammonia and pesticide emissions in the environment. Gold nanoparticals are embedded in molecular imprinted polymers to increase the sensitivity and selectivity of the measurements. SPR spectroscopy also is being employed to monitor dissolved organic carbon (DOC) content and salinity. In concert with conductivity probes and the EEM, such an array of sensors can provide a better understanding of the DOC profile. We have employed the SPR sensors to monitor DOC content around hydrothermal vent ecosystems. As Raman systems get smaller and more sensitive, the applicability to field measurements increases. We are using field portable Raman spectroscopy, with capillary waveguides, to monitor dissolved mineral content in hot springs and runoff. We are also employing Raman spectroscopy to monitor hydrothermal degradation of organics. |

|
1. “Excitation-Emission Matrix fluorescence spectroscopy in conjunction with mulitway analysis for PAH detection in complex matrices” M.L. Nahorniak and K.S. Booksh, Analyst 131 (12) 1508-1519, (2006). 2. “Determining salinity at marine temperatures using a multimode fiber optic surface plasmon resonance dip-probe,” D.J. Gentleman and K.S. Booksh*, Talanta, 68 504-515 (2006). 3. “Characterization and Quantiation of a Tertiary Mixture of Salts by Raman Spectroscopy to Simulate Hydrothermal Vent Fluids,” B.K. Dable, B. Love T.M. Battaglia, K.S. Booksh, M. Lilley, B.J. Marquardt Applied Spectroscopy, 60 (7) 773-780 (2006). 4. “Photocatalytic Degradation-Excitation-Emission Matrix (DEEM) Fluorescence for Increasing the Selectivity of Polycyclic Aromatic Hydrocarbon (PAH) Analyses,” Y.-C. Kim, M.N. Nahorniak, J.A. Jordan, K.S. Booksh*, Analytical Chemistry, 77 7679-7686 (2005) 5. “Three- and four-way parallel factor (PARAFAC) analysis of photochemically induced excitation-emission spectra,” M.L. Nahorniak, Y.-C. Kim, G.A. Cooper, and K.S. Booksh*, Analyst,130, 85-93 (2005). 6. “Fiber-optic surface plasmon resonance for vapor phase analyses”, Y.-C. Kim, S. Banerji, J.F. Masson, W. Peng, and K.S. Booksh* Analyst, 130, 838-843 (2005). |

