
What is the FDA's definition of a dietary supplement?
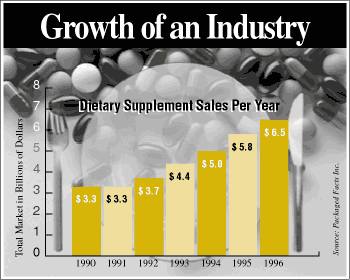
As seen from the above graph, Dietary
supplements are a growing industry. But, what exactly is a dietary
supplement? According to the FDA, a dietary supplement is a product
which is ingested as an addition to the diet, including vitamins, minerals,
herbs and plant derived products, and amino acids. They can be found
in grocery stores, health food stores, drug stores, as well as on the internet
and in the mail. These products, however, should not be confused
with drugs, which are substances used to treat or cure diseases.
Drug testing vs. Supplement testing
Because the FDA has a strict testing
policy for drugs, any that are released into the general public have been
used many times before and the side effects have been thoroughly tested.
However, dietary supplements do not undergo the same type of testing, and
are never actually approved or authorized for use. There are some
dietary supplements that have been used for 20 or 30 years, such as vitamins
and minerals, which can be considered relatively safe. If there were
horrible effects from these substances, they would have been discovered
and recalled by now. However, herbal supplements are becoming very
popular, which is dangerous because these substances have not been tested
or gone through the test of time by the public. Because these substances
are not monitored by the FDA, they should not be allowed to be advertised
to the general public.
One reason that this testing is so important for these drugs is because of their contents. Vitamins, minerals, and amino acids all have definite contents. It is possible to take calcium, vitamin C, or folic acid in pure form. The danger with plant products such as herbal supplements is that there are so many different kinds of chemicals, it is impossible to know the effects of all of these on the human body, both long and short term. Until these drugs have been proven safe and effective, it is necessary to take precautions.
How does the FDA keep America safe?
One way that the FDA is helping
to keep people safe is by requiring a list of the product's contents, and
why the ingredients are expected to be safe if used according to the package
instructions. Once the product is on the market, the only way that
it can be removed is to have the FDA prove that it is unsafe and recall
it. The FDA also requires good manufacturing processes, which means
that the products to be made would be safe and properly labeled.
Another way to protect the public is at the state legislative level.
States are allowed to ban any product that they feel is unsafe for its
residents.