

What is Ephedra and what are its main constituents used to treat?
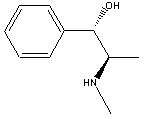
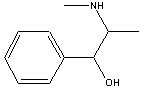
Ephedra, also know as the Chinese herb Ma Haung, is found on sandy seashores and in temperate climates of both hemispheres. It is a member of the family of herbs known as Ephedracae. Ephedra has been used in China for more than 4000 years to treat asthma and respiratory infections. The herb is available legally in all US states except Nebraska as an over-the-counter drug. Ephedra contains two main alkaloids, ephedrine and pseudoephedrine. Ephedrine is its main constituent and is a bronchodilator and stimulates the sympathetic nervous system. It acts on the air passages by relieving swellings of the mucous membrane. Pseudoephedrine is a nasal decongestant. These alkaloids can help the body to break fevers, clear blocked sinuses, treat allergic reactions, relieve general body pain, and treat low blood pressure. Because of its stimulating effects on the nervous system, ephedra is found in some popular weight loss and energy products. It suppresses the appetite and stimulates the thyroid gland which in turn stimulates metabolism. It is often combined with caffeine which increases these effects.
Ephedrine
Pseudoephedrine
What are the side effects?
Ephedra causes an increase in blood pressure and heart
rate by its stimulant properties and by its ability to constrict the blood
vessels. Side effects can therefore include palpitations, nervousness,
headache, insomnia, and dizziness. People who have high blood pressure,
prostate problems, glaucoma, heart problems, diabetes, thyroid disease,
or are taking medication for asthma, should not use ephedra.
The Safety of Ephedra
There has been much controversy over the use of ephedra because of its many side effects. The FDA has reported about 40 deaths and hundreds of serious injuries due to its use. In 1997, the FDA proposed rules prohibiting the sale of products containing more than 8 mg of ephedrine alkaloids per dose, with a maximum daily dosage of 25 mg. They also prohibited its use with caffeine or caffeine-containing herbal products. Consumers were instructed to limit its consumption to no more than seven consecutive days. A warning statement would also be required on the label. The problem is that these proposed rules were never implemented because of industry objections. Marketers continue to sell products for weight loss containing larger amounts of ephedrine combined with caffeine.
The Office of Women's Health (OWH), on the other hand,
confirmed that ephedra was safe and has many benefits. They claim
that the adverse events reported by the FDA are not consistent with published
literature. More research on its safety needs to be conducted and
because there has been many side effects, the use of ephedra should be
limited until its effects are clear.
Current Research
A controlled clinical study conducted by Harvard Medical School and Columbia Presbyterian Hospital on the safety and effectiveness of ephedrine alkaloids at marketed dosages.
CanTox study funded by the Council of Responsible Nutrition on ephedra AERs and accompanying literature.
The Ephedra Education Council (EEC) is currently conducting
a comprehensive multidisciplinary review of Ephedra by a seven-member expert
panel. They hope to present their findings at the next FDA meeting
on the safety of Ephedra.