Introduction to Requirements and Registration Procedures
ClinicalTrials.gov is a searchable web database of clinical studies of human participants. The website is maintained by the National Library of Medicine (NLM) at the National Institutes of Health (NIH).
NIH Requirements for Grants and Contracts
UD Guidance & Procedures
Why is registration required?
Federal regulations and journal publications standards require that investigators register certain clinical studies in a publicly accessible database. www.ClinicalTrials.gov (CT.gov) was created to support compliance with those requirements and standards. The diagram below depicts the different requirements and how they, in some cases, may overlap.
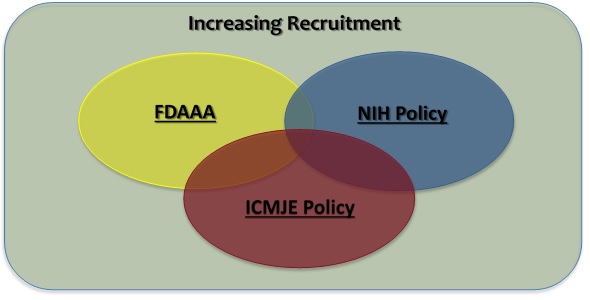
- The FDA Amendments Act of 2007 (FDAAA) requires “Applicable Clinical Trials” involving drugs, biological products, and devices subject to the FDA regulations to be registered in ClinicalTrials.gov, regardless of the funding source for the study.
- The NIH Policy on the Dissemination of NIH-Funded Clinical Trial Information (“NIH Policy”) is complementary to the reporting requirements of FDAAA and establishes the expectation that all investigators conducting clinical trials funded in whole or in part by the NIH will ensure that these trials are registered and that results submitted to ClinicalTrials.gov. .
- The International Committee of Medical Journal Editors (“ICMJE”) policy (adopted by over 1,000 journals) requires, and recommends that all medical journal editors require, registration of clinical trials in a public trials registry at or before the time of first patient enrollment as a condition of consideration for publication
- In addition, an investigator may voluntarily decide to register a study not subject to any of the requirements above (e.g., an observational study with no intervention assignment and not intended to be published in a ICMJE journal) as a way to publicly advertise the research (i.e., recruitment of research subjects). ClinicalTrials.gov supports and encourages the registration of all research studies with human subjects even if an explicit requirement does not apply.
For questions, send email to: clinicaltrials@udel.edu
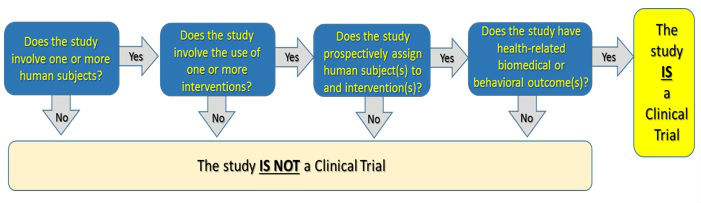
What is a Clinical Trial?
The definition of clinical trial used is slightly different depending on the requirement:
- Per FDAAA “Applicable Clinical Trials” generally include prospective interventional studies (with one or more arms) of FDA-regulated drugs, biological products, or devicesthat meet one or more of the following conditions (regardless of funding source):
- The trial has one or more sites in the United States
- The trial is conducted under an FDA investigational new drug application or investigational device exemption
- The trial involves a drug, biologic, or device that is manufactured in the United States or its territories and is exported for research
- Per NIH a Clinical Trial is research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes.
- The ICMJE defines a clinical trial as any research project that prospectively assigns people or a group of people to an intervention, with or without concurrent comparison or control groups, to study the cause-and-effect relationship between a health-related intervention and a health outcome. Health-related interventions are those used to modify a biomedical or health-related outcome; examples include drugs, surgical procedures, devices, behavioral treatments, educational programs, dietary interventions, quality improvement interventions, and process-of-care changes. Health outcomes are any biomedical or health-related measures obtained in patients or participants, including pharmacokinetic measures and adverse events.
An interactive decision tool of the FDAAA applicable clinical trials definition is offered at https://grants.nih.gov/clinicaltrials_fdaaa/ACTs_under_FDAAA.htm enabling users to identify if the FDA requirements would apply to a specific study.
For questions, send email to: clinicaltrials@udel.edu
I am the PI of a Clinical Trial. What do I do?
Once determined that a clinical trial will need to be registered in www.ClinicalTrials.gov the steps to follow are:
A. If it is my first time registering a study: How do I obtain an account with ClinicalTrials.gov?
Obtaining a username and password: If you do not have an account with ClinicalTrials.gov (i.e., no username and password), proceed to https://clinicaltrials.gov/:
- Click “Submit Studies”, and then click “How to apply for an account”.
- Towards the bottom of the page, click the link, “PRS Administrator Contact Request Form.” Complete the contact request form for the appropriate Organization (University of Delaware).
- You will receive an email from clinicaltrials.gov with the email address for the University of Delaware Protocol Registration System (PRS) Administrator account, clinicaltrials@udel.edu. Email the UD PRS Administrator account and request a username and password.
- If you do not know if you have an account or have forgotten your username, use the same steps as above to verify your username.
University of Delaware has a PRS Administrator account - do not create PRS “individual account” when registering with ClinicalTrials.gov.
- Prior to registration in ClinicalTrials.gov a UD research study must have received UD IRB approval. Pursuant to FDA Guidance, and NIH Policy the following exact statement must be included in the informed consent documents of studies to be registered in ClinicalTrials.gov:
- Once a username and password has been obtained, proceed to the ClinicalTrials.gov “PRS Login Page”, enter the Organization (UDelaware), Username, and Password.
- Complete all fields as to information related to the trial. Once all of the information has been entered in the PRS record, marked as complete, and released, an automated email will be sent to the UD PRS Administrator. Upon completion of the administrative review, which typically takes 3-5 business days, a UD PRS Administrator will approve and release the record to ClinicalTrials.gov, where the PRS team will review the record for quality control purposes prior to posting on the ClinicalTrials.gov website.
“A description of this clinical trial will be available on http://www.ClinicalTrials.gov, as required by U.S. Law. This Web site will not include information that can identify you. At most, the Web site will include a summary of the results. You can search this Web site at any time”
For basic help with using PRS, review the “Quick Start Guide” and the “PRS User's Guide” found in the “Help” section of the PRS top main menu, which is accessible after logging with your username and password.
For questions, send email to: clinicaltrials@udel.edu
How do I Submit Trial Results?
- Enter Organization (UDelaware), your Username, and your Password on the “PRS Login Page”
- Update the “Protocol Section” and release (submit) the record
- Enter the required and optional results data elements. Scientific information is submitted as four separate modules: the modules allow for the entry and display of information in a series of data tables with supporting notes, but without narrative conclusions about the results. The scientific information instructions section provides detailed information on how to prepare the submission of each module and it includes links to instructional online presentations:
- Preview, inspect, and release (submit) the record. For a description of criteria that should be addressed before releasing (submitting) the record, see “ClinicalTrials.gov Results Review Criteria”
- When the record is released, an automated email will be sent to the UD PRS Administrator. Upon completion of the administrative review, which typically takes 3-5 business days, the UD PRS Administrator will approve and release the record to ClinicalTrials.gov, where a ClinicalTrials.gov staff member will review the record for quality control purposes prior to posting the results on the ClinicalTrials.gov website
- Resources for the submission of results:
a. Ensure that the information in the Protocol Section is up-to-date before starting the Results Section; e.g., overall recruitment status, study start date, primary and study completion dates, actual enrollment, and arm and intervention information.
b. Begin results submission after the updated record has been published on ClinicalTrials.gov.
| Participant Flow | Summary of the progress of participants through each stage of a study, by study arm or comparison group. It includes the numbers of participants who started, completed, and dropped out of each period of the study based on the sequence in which interventions were assigned. The module accommodates a wide range of study designs and allows for the description of key events following study enrollment but prior to group assignment. |
| Baseline Characteristics | Summary of the data collected at the beginning of the study for all participants, by study arm or comparison group. These data include demographics, such as age and gender, and study-specific measures (e.g., systolic blood pressure prior to exercise treatment). |
| Outcome Measures and Statistical Analyses | Summary of outcome measure values, by study arm or comparison group. It includes tables for each prespecified Primary Outcome and Secondary Outcome and may also include other prespecified outcomes, post hoc outcomes, and appropriate statistical analyses. |
| Adverse Events | Summary of all anticipated and unanticipated serious adverse events and a tabular summary of anticipated and unanticipated other adverse events exceeding a specific frequency threshold. |
a. “Basic Results Data Element Definitions”, contains descriptions of each required data item. b. Results data preparation checklists, simple results templates for each module, required data, and a view of data elements in a tabular form are listed in the “Scientific Information” section of the “How to Submit Your Results” guidance on ClinicalTrials.gov. c. “Helpful Hints” contains tips on entering results data, including three examples of common study models (parallel design, crossover design, and diagnostic accuracy studies) and measure types.
For questions, send email to: clinicaltrials@udel.edu
Additional Information
- There is a link at the bottom of each page on clinicaltrials.gov to submit a ticket for help: “CONTACT NLM HELP DESK”.
- ClinicalTrials.gov may be contacted with questions or for guidance via email at register@clinicaltrials.gov.
- If the question is about a specific study record, please provide the NCT Number or the Unique Protocol ID (if an NCT Number has not yet been assigned).
- Be detailed in your request. ClinicalTrials.gov generally responds to all emails within 1 business day.
Please see ClinicalTrials.Gov Guidance & Procedures here.
For questions, send email to: clinicaltrials@udel.edu