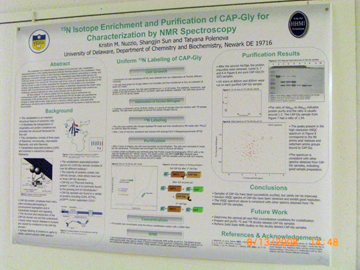
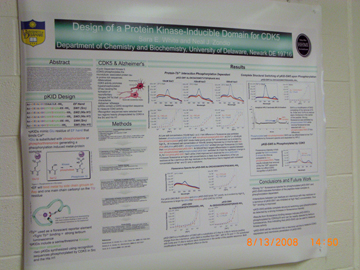
 Hybridization Studies of Human Adenylosuccinate Lyase (ASL) Wild Type/R396H Control Pair Christina H. Antonopoulos, Lushanti De Zoysa Ariyananda, and Roberta F. Colman Department of Chemistry and Biochemistry In this study,
we seek to determine the structural basis for
adenylosuccinate lyase (ASL) deficiency. Clinical symptoms of the
metabolic
disease include autism, mild to severe psychomotor retardation, muscle
wasting,
and epilepsy. Possessing two mutations for the ASL gene, ASL-deficient
patients
are unable to efficiently catalyze two beta-elimination reactions
involved in
the purine biosynthetic pathway. Most patients
are compound heterozygotes while their enzymatically normal parents are
only carriers
for one mutation. The R396H/L311V mutant pair, which was found
in a
Spanish patient, is under investigation. The ultimate goal is to
determine whether
these two disease-associated mutants form hybrids in vitro
to restore enzymatic activity. The specific activities of
the R396H and L311V mutant enzymes are 20% and 75%, respectively, that
of the
wild type enzyme. Attempting to mimic carriers of a mutant ASL gene,
here we
focus on isolating a wild type/R396H hybrid as a control group. In
addition, a
wild type/wild type hybrid mixture was tested as a pilot study. Hybrids
were
generated by mixing histidine and non histidine tagged enzymes and were
isolated using a stepwise imidazole gradient on a nickel-nitriloacetic
acid
column. Three pools of wild type/R396H hybrids were obtained using
concentrations
of 30, 45, and 60 mM imidazole. Pool two was eluted with 60 mM
imidazole and
displayed the highest specific activity (4.2 ± 0.1
µmol/min/mg). Analysis of
N-terminal sequencing data is currently being conducted to determine
the
composition of each enzyme pool. (Funded by HHMI and the McNair
Scholars
Program)
|