By tracey bryantOffice of Communications & Marketing

If it has a motor, it has a magnet — from cell phones to laptop computers. But the materials used in today's permanent magnets, produced chiefly by China, are dwindling in supply and rising in cost. University of Delaware physicist George Hadjipanayis is leading a multidisciplinary team of scientists aiming to develop the next generation of magnets to pull the U.S. industry back online.
As recently as 25 years ago, the United States ranked first in the production of magnets, George Hadjipanayis says. But then that dominance started to deteriorate.
"Now China is number one, followed by Japan, and then Europe," notes the professor and chair of the Department of Physics and Astronomy at UD. "Today, there is only one company left that makes magnets in the United States."
The strongest permanent magnets are made from neodymium and praseodymium, iron and boron (Nd(Pr)-Fe-B) as Hadjipanayis well knows. He's one of their inventors. In 1983, he published the first journal article on these magnets, which rapidly revolutionized the industry. Today, he says, the demand for such magnets is still growing, at about 15 percent per year.
"Two big areas of usage for the future are power generation and distribution. Hybrid and electric vehicles and wind turbines use lots of magnets," he notes.
However, reserves of neodymium and dysprosium — two magnetic elements in the rare earth family on the Periodic Table, used in Blackberrys to iPods to Toyota hybrid cars — are projected to last no more than 50 years, he says.

At risk of running out of the elements for its growing population, China, the producer of 97 percent of the world's supply of rare earths, has imposed export quotas and raised prices, along with international concern. Hadjipanayis has participated in recent meetings in Japan, as well as the United States and Europe, focusing on alternative magnet materials.
In 2009, Hadjipanayis won a $4.4 million federal stimulus grant from the U.S. Department of Energy's Advanced Research Projects Agency (ARPA-E) to lead a multi-institutional research project to develop advanced magnets that are less dependent on rare earth elements and twice as strong.
Magnet strength is measured in "maximum energy product" (MGOe) units. Today's permanent magnets register between 50–60 MGOe. Hadjipanayis is shooting for over 100 MGOe.
Working with him on the three-year project are chemists, materials scientists, physicists and engineers from the University of Delaware, University of Nebraska, Northeastern University, and Virginia Commonwealth University; the U.S. Department of Energy's Ames Laboratory at Iowa State University; and Electron Energy Corporation in Landisville, Pa.
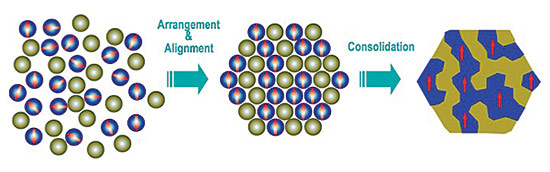
A primary focus of the team is to create nanocomposite magnets by putting together particles of neodymium as small as 50 nanometers with even tinier particles (10-20 nm) of non-rare earth elements, such as iron or cobalt.
"We're basically making magnets by putting nanoparticles together like atoms to form a solid," Hadjipanayis notes.
Professors Siu-Tat Chui and Karl Unruh in the Department of Physics and Astronomy are his co-investigators on the effort at UD, which also involves five postdoctoral researchers and several graduate and undergraduate students.
The team is assembling the particles in specific arrangements in the quest to get their magnetic spins to interact and align in the same direction, achieving optimal strength. Different particle sizes and shapes, both square and spherical, are being explored.
"It's difficult to make these nanoparticles," Hadjipanayis says. "They are highly reactive and get oxidized right away."

Iron at the size of 10 nanometers, for example, catches fire if not protected properly. To prevent that from happening in their experiments, the team uses surfactants such as oleic acid, a component of olive and vegetable oil, as part of the fabrication process. Karl Unruh is making the iron nanoparticles and colleagues from Northeastern University the iron-cobalt nanoparticles.
The team has pioneered a way to make larger quantities of the nanoparticles for research purposes, producing a slurry of "nanoflakes" with thicknesses smaller than 100 nanometers and with a crystallographic texture. Because of this, when a magnetic field is applied, the nanoflakes pile up "like a shish kebab," Hadjipanayis says. Colleagues at Virginia Commonwealth University are coating the nanoflakes with iron-cobalt to help facilitate assembly and maximize their performance.
It's a step-by-step learning process, but Hadjipanayis sees progress.
"I'm happy we're making things happen," he says.
Over the years, Hadjipanayis has witnessed the downturn in magnet research and development in the U.S., but now the tide may be turning. A rare earth mine in California recently reopened, several industry labs are starting up again, and pockets of academic research and teaching are growing, with the largest group at UD and smaller ones in Texas, Nebraska and Iowa.
"If you lose the technology, it takes time to catch up," he says. "You need to have students, and faculty to train the students, and industries for the students to work in.
"This is very high-risk research," he notes. "It takes time to find new materials, and it takes time to commercialize them. However, GM has reopened its research center, which had been closed since the 1980s, and Ford is hiring. We are going to do our best to resurrect and revive U.S. research and development on permanent magnets."