CHEM-342
Introduction to Biochemistry
Name ______________________________
Midterm Examination - Individual
Part
Friday, 23 March 2001
H. B. White - Instructor
Important - Please read this before you turn the page.
-
Write your name on each page
-
This part of the midterm examination is worth 75 points distributed
as follows:
A. (15 points) Three Short Answer Questions.
B. (60 points) Four Problem and Short Essay Questions.
-
You may refer to your notes, course reader, handouts, or
graded homework assignments. Textbooks or reference books cannot be used.
-
This examination will assess your learning, problem-solving
skills, and ability to communicate clearly. It is intended to be challenging
even to the best students in the class.
-
Writing reflects how you think. Among the "right answers"
I will read for the following questions, some will be better than others
because they show greater depth of understanding, avoid extraneous or inaccurate
information, provide a more logical structure, use appropriate examples,
and chose words with precision. Better quality answers will receive higher
marks. Therefore organize your thoughts before you write.
-
Strive to write not that you may be understood, but rather
that you cannot possibly be misunderstood. Stream of consciousness answers
are rarely well organized or clearly presented.
-
Have a relaxing and safe Spring Break.
Exam Statistics
Number of students
26 Ave.
51.0
Range 28 - 67 (out of 75 points)
Number of groups
6 Ave.
19.7
Range 16 - 23 (out of 25 points)
Class Totals
Ave. 70.7
Range 40 - 89 (out of 100 points)
Part I Three Short Answer Questions. (5 Points
each)
A. What is the reaction of [Fe(CN)6]3-
with oxyhemoglobin.
B. Why does the addition of oxygen to a solution
of methemoglobin and deoxyhemoglobin cause a change in the electrochemical
potential?
C. In plain words, what is the meaning or significance
of the term (1-Vp) in Svedberg’s equation?
Part II Problems and Short Essays
1. (15 Points) Given the premise of this course that
we never fully understand anything we study, each of us (including the
instructor) should have unresolved learning issues associated with each
of the articles we have studied. Satisfying those learning issues should
lead to deeper understanding, new perspectives, and greater appreciation
of the articles and of science in general. This course promotes skills
in identifying areas of personal ignorance and the transformation of that
"knowledge" into effective ways of learning new information.
In the space provided, list your most important unresolved
learning issue. It may relate to any of the articles we have discussed
or to issues involving several articles. Remember, at this stage
you should be beyond defining words as learning issues and be probing central
themes, underlying concepts, and issues of significance. I am looking for
a well-articulated, substantive learning issue (preferably not from the
lists handed out) and a clear explanation of why you think it is important
for you to resolve. (Ideally you should be able to write this without referring
to your notes.)
Learning Issue:
Importance:
2. (15 points) The diagram below
represents the titration of indigo carmine with dithonite as it was done
in class on 12 February.
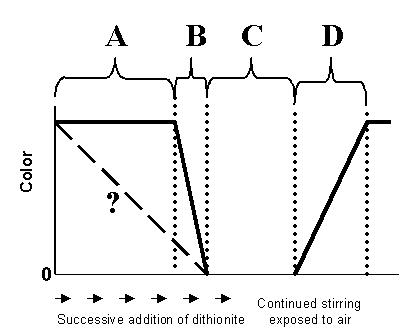
A. Explain what is happening chemically in regions A,
B, C, and D.
Region A.
Region B.
Region C.
Region D.
B. Does your answer for regions A and B explain why the
color does not decrease as depicted by the dashed line? Explain.
3. (15 Points) The following figure is intended to depict
what is going on in Section 10 of the Stokes article. However, words describing
the relative speed with which the various reactions occur are omitted (blanks).
A. Provide the missing words to complete the
diagram.
B. In the space
below the figure, describe in words what the figure explains. Please feel
free to draw a figure of what Stokes described to accompany your description.

4. (15 Points) The articles you have
read so far this semester were selected with a purpose. Compare, contrast,
and reflect on the Conant (1923) and Svedberg & Fåhraeus (1926)
articles as they relate, respectively, to the Stokes (1864) and Zinoffsky
(1886) articles.
Return to Hal
White's Home Page or Departmental
Home Page.
Posted: 25 March 2001
by Hal White
Copyright 2001, Department
of Chemistry and Biochemistry, University of Delaware