 Boston Convention Center Marquis |
 EB2015 EXPERIMENTAL BIOLOGY MEETINGS Boston, MA March 27-APRIL 1, 2015  |
 Boston Night Skyline from Wharf |
 Boston Convention Center Marquis |
 EB2015 EXPERIMENTAL BIOLOGY MEETINGS Boston, MA March 27-APRIL 1, 2015  |
 Boston Night Skyline from Wharf |
The University of Delaware group included four faculty and 9 undergraduates.
 From left to right: Gabe, Becky, Lauren, Brooke, Matt, Molly, Andre, Hannah, Tom (in back) |
||
| Prof.
Hal White, Chem &Biochem Prof. Dave Usher, Biol. Sci. Prof. Seung Hong, Biol Sci Prof. Gary Laverty, Biol Sci |
Hannah Anderson Andre Freligh Matthew Fischer Lauren Genova Gabriel Gregorzak |
Brooke Palus Molly Peters Rebecca Pollak Thomas Rivas |
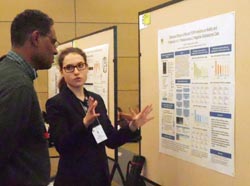 Hannah Anderson Winner of an Honorable Mention Award in the ASBMB Undergraduate Poster Competition
|
Differential
Effects
of FAK and FGFR Inhibitors on Motility and Proliferation of L1-Positive
versus
L1-Negative Glioblastoma Cells Hannah
Anderson and Deni
Galileo Department
of
Biological Sciences, University of Delaware, Newark, DE 19716 Glioblastoma
multiforme (GBM), a highly
invasive astrocytoma, is the deadliest form of brain cancer. Glioblastoma cells express the L1CAM cell
adhesion protein (L1), which is cleaved at the cell surface and binds
to both
integrins and fibroblast growth factor receptors (FGFRs) in an
autocrine/paracrine manner. Focal
adhesion kinase (FAK), which is activated by L1-integrin interactions,
and FGFR
are tyrosine kinases that initiate signaling cascades stimulating GBM
motility
and proliferation. We hypothesized that
inhibitors of FGFR and FAK would have differential effects on the
motility and
proliferation of T98G glioblastoma cells expressing L1 compared to
those with
attenuated L1 expression. Short hairpin
RNA delivered by a lentiviral vector was used to block L1 expression. Cell motility was measured through
quantitative time-lapse microscopy, and proliferation was measured by
cell
cycle analysis and quantified as the population of cells in S phase. FAK inhibitor Y15 had a greater effect in
L1-positive versus L1-negative cells on motility (-45.3% vs. -5.6%) and
S phase
population (-6.5% vs. -3.5%). FAK
inhibitor PF431396 also produced a greater reduction in motility
(-57.2% vs.
-15.4%) and S phase population (-11.7% vs. -6.2%) in L1-positive versus
L1-negative cells. An inhibitor of FGFR,
PD173074, reduced motility (-37.8% vs. +2.3%) and S phase population
(-8.6% vs.
-0.4%) in L1-positive cells only. We
conclude that inhibitors of FAK and FGFR drastically decrease
L1-stimulated motility
and proliferation in glioblastoma cells, indicating that they have
chemotherapeutic potential for GBM tumors expressing L1.
Research support was provided by the Delaware
Governor’s Bioscience Fellowship and the Center for Advanced Technology. |
|
Cooperation at the Molecular Level Affects Your Performance and Health Andre
Freligh and John R.
Jungck Many biological macromolecules are composed of subunits that function synergistically due to cooperativity. I have generated a spreadsheet model of the Hill Equation for the Biological Excel Simulations and Tools for Exploratory Experiential Mathematics (ESTEEM) modules page:http://bioquest.org/esteem/esteem_result.php in order for users to interactively develop a sense of nonlinear behavior of such macromolecules. This software is accessible to students and professors both nationally and internationally. Originally, the Hill equation represented the cooperative binding process of oxygen to hemoglobin molecules. Although Hill’s model is not precise for modeling all forms of biological cooperativity, it is accurate enough that it is frequently utilized to describe the cooperative behavior of enzymes. In the model the parameters of the Hill equation are adjustable so that students can monitor the effects of adjusting such parameters. Additional spreadsheets will incorporate real world data about the binding of oxygen to hemoglobin under various conditions, the binding of ligands to enzymes, and genetic repressors whose data also fit the Hill equation. Medically, this model is relevant to the cooperativity of hemoglobin during intense anaerobic physical activity and is important to understanding the development of drugs for treating diabetes, sickle cell anemia, and thalassemia. |
|
Department
of Biological Sciences, University of Delaware, Newark, DE 19716
8J16 and 9E6 are two independent allelic point mutations in Drosophila melanogaster autophagy-specific gene 18a (atg18a) located in introns three and four, respectively, of its 5 exon locus at 66B11. The atg188J16 and atg189E6 intronic mutations are not in sequences expected to disrupt transcript processing, yet these mutations cause pupal lethality and neurodegenerative phenotypes by an unknown molecular mechanism. The purpose of this research is to examine the genotype-phenotype relationship of atg188J16and atg189E6on neuronal maturation using the Drosophila eye as a model. RT-PCR analysis revealed no detectable effects on atg18 mRNA splicing in either mutant background. In adults, atg188J16 and atg189E6 homozygous mutant eyes are small and display a rough eye phenotype. To understand the basis of this phenotype, homozygous mutant larval imaginal eye discs were examined. ELAV, a pan neuronal marker, showed that photoreceptors differentiate normally, but those that differentiate early are being lost by apoptosis as determined by the increased cleaved caspase 3. These mutations also caused significant death at the morphogenetic furrow, which may result in a smaller pool of photoreceptor progenitors. Hence, autophagy may have roles in both photoreceptor differentiation and maintenance and its absence leads to apoptotic death. Senseless and Prospero expression showed that photoreceptors R8 and R7, respectively, are more refractory to apoptosis as these photoreceptors predominate near the optic stalk. This suggests autophagy is more important for the maintenance of photoreceptors R1, R2, R3 R4, R5 and R6 than R7 and R8. Acknowledgements given to the Howard Hughes Medical Institute and University of Delaware Undergraduate Research Program for financial support. |
|
Investigating
the Binding
Affinity of Nod2 and Soluble Bacterial Cell Wall Dimers Department of Chemistry
and
Biochemistry, University of Delaware, Newark, DE 19716 The innate immune system is
the body’s first line of defense against pathogens. The innate immune
system is
triggered by pathogen associated molecular patterns (PAMPs) that are
recognized
by pattern recognition receptors (PRRs) such as Toll-like receptors
(TLRs) and
Nod-like receptors (NLRs). This research project focuses on providing a
better
understanding of how the innate immune system senses and responds to
the
presence of bacteria. Specifically, our group is interested in the
relationship
between the nucleotide-binding oligomerization domain-containing
protein 2
(Nod2), an NLR protein found in the cytosol of mammalian host cells,
and
muramyl dipeptide (MDP), the smallest bacterial cell wall fragment
known to
elicit an immunological response. When Nod2 is mutated, the signaling
pathway
becomes disrupted and uncontrollable inflammation arises, leading to
chronic
inflammatory bowel disorders such as Crohn’s disease. To discover how
to
better treat these diseases, it is imperative to learn more about
how Nod2
and MDP interact, a mechanism which is currently unknown. The Grimes
Lab has
previously shown that Nod2 binds to MDP in vitro; however,
research
suggests that a heightened immunological response may be elicited in a
host if
molecules containing multiple MDP’s are used, suggesting multivalency
is at
play. To test this hypothesis, a variety of novel MDP dimers were
synthesized
to be assessed via in vitro SPR
binding assays, as well as through cell-based assays. |
 Gabriel Gregorzak
|
Fueling the Interdisciplinary Flame:
Exploring Plant-Based Alternative Fuels
in the Undergraduate Laboratory Gabriel Gregorzak1, Mark Baillie1, Jacqueline Fajardo1 & Alenka Hlousek-Radojcic2 1Department of Chemistry & Biochemistry, University of Delaware, 2Department of Biological Sciences, University of Delaware A laboratory
module intended for an integrated course was
developed to highlight relevant and interrelated concepts described in
both introductory
biology and general chemistry with an enrollment of nearly 500
students. The
ever-present depletion of petroleum energy reserves is an ongoing
societal
concern. Concomitantly, there is growing interest in identifying
natural
sources of alternative fuels and optimizing the efficiency of their
use. To
enhance student awareness of alternative fuel availability from natural
resources, we have developed a lab centered on the production of
biofuels
produced from seed-oil extracts. Oil was physically extracted from a
variety of
seed types including pumpkin, sunflower, walnut, & flax, and
utilized an
efficient, small-scale, and green approach. The physical extraction
technique
replaced commonly used organic solvent extraction methods used in many
academic
and educational labs. Extracted oil was then subject to
transesterification to
yield the biofuel product. The energy content of this biofuel was
measured and
compared to ethanol using calorimetric analysis. This distinctive
laboratory
experience will allow large numbers of freshmen students to recognize
the
broader implications of their curriculum beyond the boundaries of the
classroom.
|
 Brooke Palus
|
Brooke Palus and Erica M. Selva O-xylosyltransferase
(Oxt) is a transmembrane glycosyltransferase that initiates the first
step in
heperan and chrondroitin sulfate (HS and CS) proteoglycan biosynthesis.
HSPGs
and CSPGs are abundant in the extracellular environment of many
tissues, such
as articular cartilage in humans. In this tissue, HSPGs and CSPGs are
essential
regulation of signaling pathways that maintain tissue homeostasis. If
homeostasis is not preserved, joint tissue will degrade and
osteoarthritis (OA)
can occur. Elevated levels of circulating Oxt have been detected in
human OA
patients suggesting shed Oxt may have an extracellular function. Little is known about Oxt behavior or
function once it is released from the plasma membrane. Understanding
the role
of extracellular Oxt may provide further insight to how cell signaling
is
disrupted in OA patients. Cell
culture experiments have
shown that Oxt, normally located in the Golgi and the endoplasmic
reticulum
(ER), can be shed by proteolysis activity and move into the media. It
is
therefore likely that under the appropriate conditions in vivo Oxt
will
also be shed and become an extracellular protein. The overall aim of
this
project is to determine if Oxt released from expressing cells in
vivo and
what, if any, function extracellular Oxt has in organismal development.
The
data collected thus far shows localization of Oxt to the Golgi and the
ER where
it could have an active role in HSPG and CSPG biosynthesis. Movement of
Oxt
from expressing cells to non-expressing cells is observed in the
imaginal wing
disc of Drosophila larva.
Extracellular staining shows that Oxt is found predominantly on the
apical surface
and can be detected in the peripodial space suggesting it is released
from
expressing cells. Western analysis of Drosophila hemolymph indicated Oxt is
released into hemolymph, as observed in humans. The
results suggest that cleaved Oxt is found in the
hemolymph of Drosophila and future
studies will
explore the potential function of shed Oxt. |
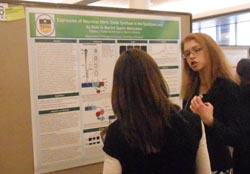 Rebecca Pollak
|
Expression of
Neuronal Nitric Oxide Synthase (nNOS) in the Extratesticular Pathway
and Its
Role in Murine Sperm Maturation Rebecca
Pollak
and Patricia A.
Martin-DeLeon Department
of Biological Sciences,
University of Delaware Neuronal
nitric oxide synthase (nNOS) is one
of two constitutive enzyme variants responsible for the production of
nitric
oxide (NO) from L-arginine in mammalian cells. This membrane-associated
protein
has been shown to be activated by Ca2+ and to interact with
Plasma
Membrane Calcium ATPase 1 and 4 (PMCA1 and PMCA4), which negatively
regulate
it. PMCA4 is the major calcium efflux pump in murine sperm (Wennemuth
et al.
2003), where its deletion leads to loss of motility and ultimately male
infertility. NO is an important second messenger, and is required for a
variety
of sperm functions, including motility and fertilizing ability (Ramya et al.
2011).
Recently, the DeLeon Lab has shown the expression of PMCA4 in the
murine epididymis.
However, no work has been done on the expression of nNOS in the
extratesticular
pathway of any mammalian species. Therefore, we set out to investigate
the
expression pattern of nNOS in the post-testicular pathway and its role
in sperm
maturation. Here, we show the presence of nNOS in all three regions
(caput,
corpus, and cauda) of the murine epididymis in the basal and apical
regions of
the epithelial lining, via immunofluorescence. Western blotting
confirmed the
expression of nNOS throughout the epididymis, and showed regional
differential
expression: there was significantly (P = 0.005) higher amounts in the
corpus as
compared with the caput and cauda. In the epididymal luminal fluids
(ELF), nNOS
was found to be significantly (P = 0.027) higher in that from the
caudal region
as compared with those from the caput and corpus. Similarly, caudal
sperm had
significantly (P = 0.013) higher expression of nNOS than that in caput
sperm.
When ELF (combined from all regions) was fractionated via
ultracentrifugation,
Western analysis showed that nNOS was exclusively present in the
epididymosomes
(membrane vesicles). Following co-incubation of caudal sperm and ELF,
epididymosomal nNOS was transferred to the sperm surface, as detected
by flow
cytometry. Our finding of sperm acquisition of nNOS from ELF in vitro, as well as elevated levels in
caudal sperm, is consistent with the presence of the PMCA4-nNOS
interactome in
epididymosomes and also a role for nNOS in epididymal sperm maturation. |
|
Biochemical
Characterization of NOD1, an Innate Immune
Receptor Thomas Rivas,
Mackenzie Lauro, Walter Drake, Catherine
Grimes, & Brian
Bahnson Department of
Chemistry and Biochemistry,
University of
Delaware |
 Hannah receiving her Honorable Mention Award. |
 Boston Convention Center Floor where posters were displayed. |
 Waiting for the Subway at Boston South Station. |
 Sunset over
Boston Commons. Sunset over
Boston Commons. Yes, that is snow in the background. |
|
 View of Boston Commons toward the Prudential Building. |
 Snow still piled high in Boston Commons after a record winter snowfall. |
 ASBMB Undergraduate Poster Competition. |
 Gabe, Andre, and
Hannah at ASBMB Undergraduate Poster Competition.
|
|
 Lunch break for Matt and Brooke. |
 \ \Dinner at Ristorante Limonell after our arrival in Boston. |
 Lauren and Gabe go Western |
 Doug Kenny, Hal White, and Lauren Genova at Durgin Park. |
|
 A highlight of the meetings was to hear back-to-back plenary talks by Nobelists Stanley Pusiner and Eric Kandel. |
 Dinner at Ruth's Steak House.. |
 Brooke, Becky, and Lauren at Quincy Market. |
 UD Alumni
dinner at Durgin Park Restaurant.
|
|
 UD Alumni: Aparna Sapra, Sarah Martin, Doug Kenny,Sarah's husband, and Justin Teesdale. |
 UD Alumni dinner at Durgin Park Restaurant. |
 Steve Foltz, Mandy Simons, Isaac Hubner, Hal White, and Tom Rivas. |
 Molly & Isaac
Hubner and Evan
& Lauren Lebois and spouses at Durgin Park.
|
|
 Lauren and Evan Lebois, Allen Tseng, and Eric Borer at Durgin Park. |
 Aparna Sapra, Sara Martin, Doug Kenny, Kyle Martin, and Justin Teesdale at Durgin Park. |
 Hal White and Mike Cox at Durgin Park. |
 Eric Borer, ?, Allen
Tseng, Courtney Ngai, and Gabe Gregorak at Durging Park.
|
|
 Brooke, Tom, and Becky on Washington Srtreet |
UD
Alumni attending the dinner: Michael Cox (BA Bioloy 1974), Marilee Benore (PhD Chemistry 1986), Daniel Dries (BS Biochemistry 2000), Amanda Simons (BS Biochemistry 2001), Isaac Hubner (BS Biochemistry 2001), Laura Maliszewski (BS Biology 2001), Evan Lebois (BS Biochemistry 2007), Allen Tseng (BS Biology 2007), Courtney Ngai (BS Biochemistry 2011), Eric Borer (BS Biochemistry2011), Steve Foltz (BS Biochemistry 2011), Ed Miracco (PhD Chemistry 2011), Justin Teesdale (BS Chemistry 2013), Sara Martin PhD Chemistry 2014), Doug Kenny (BS Chemistry 2014), Aparna Sapra (PhD Chemistry 2014). . |
 Drs. Usher,
White, and Laverty discussing dinner plans.
|
||
The trip to the Experimental Biology
2015 Meetings
in Boston was organized by the University of Delaware HHMI
Undergraduate
Science Education Program with additional support from travel grants
from
the American
Society for Biochemistry and Molecular Biology. The HHMI
Undergradaute Science Education Program, Charles Peter White
Fund, Undergraduate Research
Program, NIH, NSF, supported
research by individual students.