|
Conformational Effects
of Protein Phosphorylation
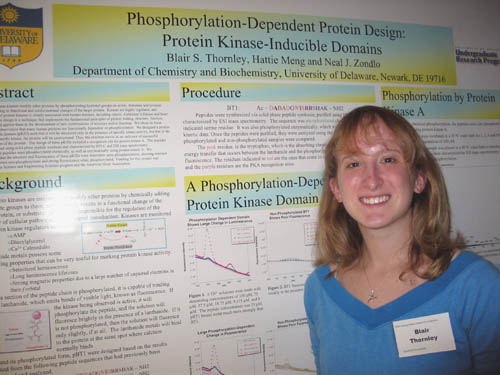
Agata Bielska and Neal Zondlo Department of Chemistry and Biochemistry Phosphorylation-induced conformational change is an important tool used by cells to regulate metabolic pathways, signal transduction pathways, and transcription. The objective of this research is to characterize the specific conformational effects of protein phosphorylation using the proteins tau and cJun as a model. Hyperphosphorylation of tau has been shown to cause its dissociation from microtubules and subsequent precipitation into neurofibrillary tangles (NFTs) in Alzheimer’s disease. In order to study mechanisms by which phosphorylation could lead to NFT formation, various tau-derived peptides were synthesized. Phosphorylated and non-phosphorylated versions of the peptides were then analyzed using circular dichroism (CD) and NMR spectroscopy. Significant conformational changes were found in regions which, when hyperphosphorylated, are associated with NFT formation. These conformational changes were consistent with an increase in type two polyproline helix (PPII) character. The oncoprotein, cJun, is a transcription factor regulated by phosphorylation. Peptides derived from cJun were synthesized and analyzed in non-phosphorylated and phosphorylated forms. Upon phosphorylation, significant conformational changes were observed favoring PPII formation. The magnitude of these changes increased as the number of phosphorylation sites increased. Supported by an HHMI . Winner of the Sigma Xi Award for the best oral presentation at the 2005 Undergraduate Research Symposium. |



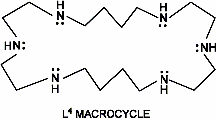
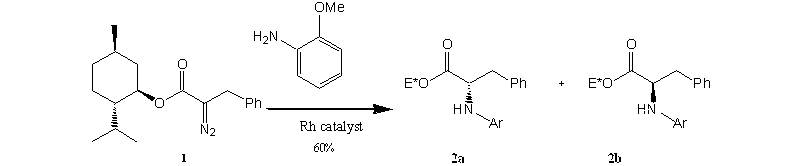
 Neopentyl Derivative of L*
Neopentyl Derivative of L*