Home Page: Taber home page.
Chem 334
Department of Chemistry and Biochemistry
University of Delaware
Friedel-Crafts Acylation
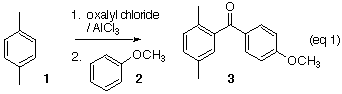
Friedel-Crafts Synthesis of an Unsymmetrical Benzophenone
Chlorocarbonylation of an arene followed by the addition of another arene, illustrated by p-xylene 1 and anisole 2, leads smoothly to the unsymmetrical benzophenone 3. The product ketone can be purified by recrystallization or by column chromatography

Experimental Section:
Following the procedure for chlorocarbonylation, combine 212 mg of p-xylene and 7 mL of CH2Cl2 in a 25 mL Erlenmeyer flask. Stir in an ice/water bath, then add 2.1 g of a 10 weight percent solution of oxalyl chloride (CAUTION: Corrosive!) in CH2Cl2. Add 0.53 g of solid AlCl3 (CAUTION: Corrosive!) over 5 min. Remove from the ice bath, and let stir 30 min.
Add 0.22 g of anisole in 2 mL of CH2Cl2 dropwise over 5 min. Let stir for 1 h. Quench cautiously by adding the reaction mixture to cold water in a separatory funnel. Separate the CH2Cl2 layer, and extract the aqueous layer with two more 10 mL portions of CH2Cl2. Dry the CH2Cl2 over Na2SO4. Set aside 0.5 mL of the CH2Cl2 solution for TLC, and evaporate the rest of the CH2Cl2 solution onto 0.5 g of flash silica using the rotary evaporator.
TLC: using 5% EtOAC/hexane, you should see two UV-absorbing spots that move, with more material at the baseline. The ketone that you want is the more slowly-moving spot.
Column: Prepare a chromatography column using 5 g of flash silica gel. Add your sample to the top, followed by a layer of sand. Elute the column with 20 mL of 1% EtOAc/hexanes, 20 mL of 2% EtOAc/hexanes, and 20 mL of 5% EtOAc/hexanes. Collect 5 mL fractions in test tubes,1 and check them by TLC. You should be able to spot at least four fractions across the bottom of a TLC plate. Combine the fractions that contain your pure ketone, and concentrate them on the rotary evaporator. The residue after evaporation should be solid. Filter it with cold hexane, collecting the crystalline product on a Hirsch funnel. Record the melting point, weight and physical appearance of your product, and calculate the yield. The melting point should be about 86o.
Notes:
1. This could mean as many as 60 5 mL fractions. You do not have 60 test tubes. Collect ten fractions at a time, and check the TLC of each tube. If it does not have your desired product, rinse the contents of the tubeinto a 250 mL Erlenmeyer flask, and reuse the tube. When you are sure that you have found your product, pour the contents of the 250 mL Erlenmeyer flask into the waste receptacle designated for it.