The Retort

Mosher’s Acid
My previous blog post gave an example of resolving enantiomers by converting a racemic mixture to a mixture of diastereomers. This is a general concept (as described in your text in Section 4.9) and is not limited to salt formation.
We also mentioned in class the concept of “kinetic resolution”: if two enantiomers react at different rates with an a chiral reactant or catalyst, then it is possible to selectively convert one enantiomer to product and leave the other reactant untouched.
The use of Mosher’s acid as a derivatizing agent for alcohols and amines demonstrates several of these concepts. Mosher’s acid is available as a single enantiomer, and can convert alcohols and amines to the corresponding esters and amides:
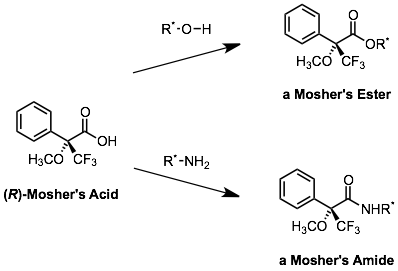
Because the Mosher’s acid is enantiomerically pure, a racemic (50:50 d:l) or scalemic (not 50:50) mixture of alcohols or amines can be converted to a mixture of diastereomeric esters or amides. Mosher’s esters and amides are often used to determine the enantiomeric purity of a scalemic mixture of alcohols or amines. Most commonly, a technique called Nuclear Magnetic Resonance spectroscopy (which will be covered in CHE322) can be used to detect, and measure the amounts of, two diastereomeric Mosher’s derivatives. Because the products are diastereomers, they have different physical and chemical properties, and that includes their appearance in an NMR spectrum.
In the example shown below, only one of the two possible diastereomers were detected, indicating that the starting alcohol was enantiomerically pure to the limits of detection. This sequence also demonstrates the use of enzymes to perform an enantioselective reaction (here, reduction of a ketone to an alcohol).
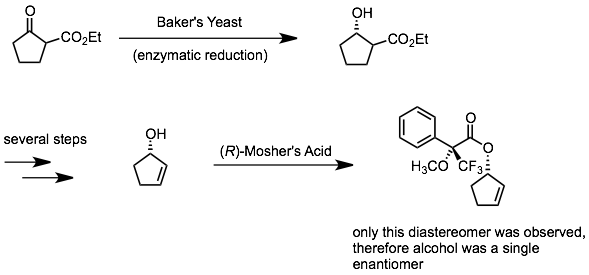
However, when preparing the Mosher’s ester or amide, you need to get very high conversion (essentially 100%) to guarantee that the ratio of ester/amide diastereomers accurately reflects the ratio of alcohol/amine enantiomers. The reason is that, as conversion is less and less complete, kinetic resolution of the starting alcohol or amine may be occurring. If, say, the d- enantiomer reacts with R-Mosher’s acid faster than the l-,the Mosher’s ester/amide will contain a higher ratio of the d- enantiomer’s Mosher derivative unless you get all of the l- to react as well.
Finally: how is Mosher’s acid available as a single enantiomer? It is resolved via diastereomeric salt formation, using (R)-1-phenylethylamine:

References:
Mosher, H. S. et al. J. Org. Chem. 1969, 34, 2543.
Rauk, A. et al. Can. J. Chem. 1990, 68, 1559.
Monday, October 22, 2012
