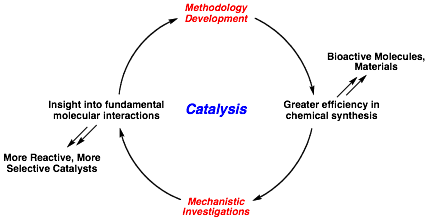
Our research is centered on the discovery of new catalytic, stereoselective methods for organic synthesis that utilize the power of transition metal catalysts. Our goal is to enable greater efficiency and novel bond constructions in the synthesis of stereochemically complex molecules, with applications that extend to natural product synthesis, drug discovery and process chemistry. The development of these methods also provides a platform for mechanistic investigations, which will enable insight into questions of fundamental reactivity and catalyst design.
Metal Catalysis of Cationic Intermediates
In this aspect of our research, we are excited to develop transition metal catalysts that enable novel reactions and enantioselective transformations of cationic intermediates, such as iminium and oxocarbenium ions.
Enantioselective Additions to Oxocarbenium Ions
We are developing metal-catalyzed, enantioselective additions to cyclic oxocarbenium ions. These additions enable efficient preparation of enantioenriched alpha-substituted oxygen heterocycles
from readily available racemic acetals. For example, we have developed the highly enantioselective addition of terminal alkynes to isochroman acetals catalyzed by chiral copper/bis(oxazoline) catalysts.
Metal-Catalyzed Reactions of Iminium Ions
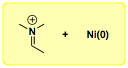
We have also discovered a novel nickel(0)-catalyzed cyclization of
N-benzoylaminals to form isoindolinones,
which represent an important class of bioactive molecules. This reaction offers novel entry to 3-substituted isoindolinones and likely proceeds via an alpha-amido alkyl nickel intermediate.
Cross Coupling Reactions of Non-Traditional Substrates
One project in our group is focused on the use of non-traditional substrates in cross coupling reactions. Extremely powerful methods, such as the Heck, Suzuki and Buchwald–Hartwig reactions, have been enabled by the activation of weak carbon–halide and carbon–oxygen bonds using transition metal catalysts. We are investigating the use of less traditional substrates for similar cross couplings. By targeting readily available, inexpensive starting materials, we hope to enable more efficient or “greener” access to coupling products than traditional cross couplings allow. Our recent work in this area includes a nickel-catalyzed Heck cross coupling of aryl pivalates with olefins, which is one of the first times a non-org
anometallic partner has been used in cross couplings via activation of strong C–O bonds. This reaction enables the preparation of 1,2-disubstituted olefins from a phenol derivative without the necessity of an expensive triflate group.