Publications
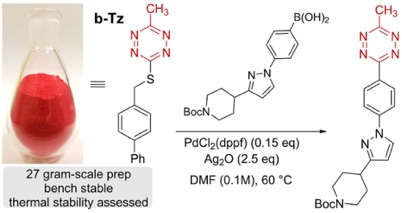
122) Lambert W, Tsang S, Hillman A, Wang C, am Ende C, Fox J. Second Generation Catalyst System for Ag-mediated Liebeskind-Srogl Coupling of Tetrazine Thioethers with Arylboronic Acids. J. Org. Chem. Accepted. ChemRxiv. 2025; doi:10.26434/chemrxiv-2025-nbrrh
121) Pol, M.; Gao, H.; Fox, J. M.; Jia, X.* "Integrin–Binding Peptide Cooperates with Soluble TGF–β1 to Promote Metastasis in an Engineered Model of Prostate Cancer" ACS Biomat. Sci. & Eng., 2025, in press.
120) Neglia, S. E.; Tallon, A. M.; am Ende, C. W.; Thorpe, C.; Fox, J. M. Ascorbate Peroxidase (APEX2) Activates Dihydrotetrazine Oxidation for Rapid Bioorthogonal Chemistry in Living Cells. ChemRxiv. 2025; doi:10.26434/chemrxiv-2025-prsq2
119) Ratna, S.; Pradhan, L.; Vasconcelos, M. P.; Acharya, A.; Carnahan, B.; Wang, A.; Ghosh, A.; Bolt, A.; Ellis, J.; Hyland, S.; Fox, J. M.; Kloxin, A.; Neunuebel, R.; Grimes, C. L. “The Legionella pneumophila peptidoglycan recycling kinase, AmgK, is essential for survival and replication inside host alveolar macrophages”, BioRxiv, doi: 10.1101/2025.03.21.644609.
-
118)Kim, J.; Xu, Y.; Lim, J. H.; Lee, J. Y.; Li, M.; Fox, J. M.; Vendrell, M.; Kim, J. S. Bioorthogonal Activation of Deep Red Photoredox Catalysis Inducing Pyroptosis. J. Am. Chem. Soc. 2025, 147, 701-712. DOI: 10.1021/jacs.4c13131

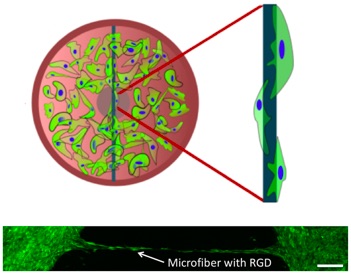
117) Ramaraj, P. K.; Pol, M.; Scinto, S. L.; Jia, X.*; Fox, J. M.* Covalent Attachment of Functional Proteins to Microfiber Surfaces via a General Strategy for Site–Selective Tetrazine Ligation. ACS Appl. Mater. & Interfaces, 2024, https://doi.org/10.1021/acsami.4c12609.
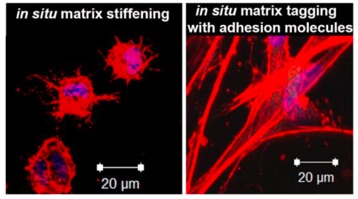
116) Gao, H.; Pol, M.; Makara, C. A.; Song, J.; Zhang, H.; Zou, X.; Benson, J. M.; Burris, D. J.; Fox, J. M ; Jia, X.* "Bioorthogonal Tuning of Matrix Properties during 3D Cell Culture to Induce Morphological and Phenotypic Changes" Nat. Protoc., 2024, in press, DOI : 10.1038/s41596-024-01066-z.
115) Gao, H.; Zhang, T.; Langenstein, M. G.; Xie, W.; Udan, S.; Zhang, Z.; Saven, J. G.; Bai, S.; Pochan, D. J.; Fox, J. M. ; Jia, X.* "High Molecular Weight Protein–Like Semiflexible Chains via Bioorthogonal Polymerization of Coiled–Coil Peptides" Macromolecules, 2024, 57, 20, 9585–9594. DOI: 10.1021/acs.macromol.4c01866.

-
114)Devaraj, N.; Fox, J. M.; Lei, X.; Wang, Q. Next-Generation Organic Chemistry for Labeling and Imaging. J. Org. Chem. 2024, 89, 14609–14611. DOI: 10.1021/acs.joc.4c023.
113) O’Brien, J. G. K.; Conway, L. P.; Ramaraj, P. K.; Jadhav, A. M.; Jin, J.; Dutra, J. K.; Evers, P.; Masoud, S. S.; Schupp, M.; Saridakis, I.; Chen, Y.; Maulide, N.; Pezacki, J. P.; am Ende, C. W.; Parker, C. G.; Fox, J. M. Mechanistic Differences between Linear vs. Spirocyclic Dialkyldiazirine Probes for Photoaffinity Labeling. Chem. Sci. 2024, 15, 15463–15473. DOI: 10.1039/D4SC04238G
112) Fang, Y.; Hillman, A.S.; Fox, J. M. Advances in the synthesis of bioorthogonal reagents—s-Tetrazines, 1,2,4-Triazines, Cyclooctynes, Heterocycloheptynes, and trans-Cyclooctenes. Top. Curr. Chem. 2024, 382, 15. https://doi.org/10.1007/s41061-024-00455-y.
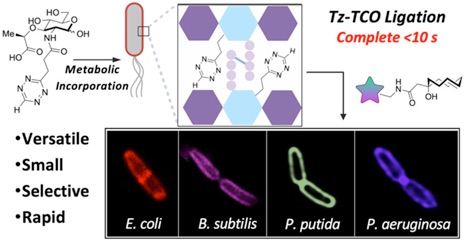
111) Hillman, A.S.; Hyland, S.N.; Wodzanowski, K.A.; Moore, D.L.; Ratna, S.; Jemas, A.; Sandles, L.M.; Chaya, T.; Ghosh, A.; Fox, J.M.; Grimes, C.L.; Minimalist Tetrazine N-Acetyl Muramic Acid Probes for Rapid and Efficient Labeling of Commensal and Pathogenic Peptidoglycans in Living Bacterial Culture and During Macrophage Invasion. J. Am. Chem. Soc. 2024, 146, 6817–6829.
110) Xu, M.; Ma, X.; Pigga J.E.; Zhang H.; Wang, S.; Zhao, W.; Deng, H,; Wu, A. M,; Liu, R.; Wu, Z.; Fox, J. M.; Li, Z. Development of 18F-Labeled Hydrophilic trans-Cyclooctene as a Bioorthogonal Tool for PET Probe Construction. Chemical Communications 2023, 59, 14387. doi:10.1039/D3CC04212J
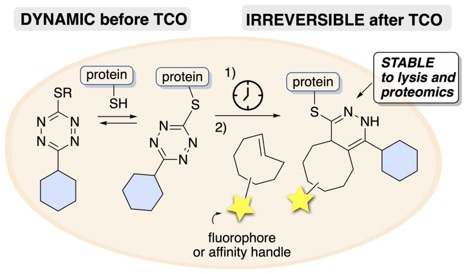
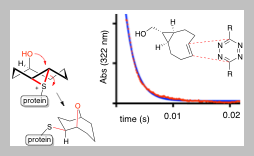
109) Tallon A, Xu Y, West G, am Ende C, Fox J. Thiomethyltetrazines are Reversible Covalent Cysteine Warheads whose Dynamic Behavior can be "Switched off” via Bioorthogonal Chemistry Inside Live Cells. J. Am. Chem. Soc. 2023, 145, 29, 16069–16080.
https://pubs.acs.org/doi/10.1021/jacs.3c04444
108) Zou, X.; Zhang, H.; Benson, J. M.; Gao, H.; Burris, D. L.; Fox, J. M.; Jia, X.* "Modeling the Maturation of the Vocal Fold Lamina Propria Using a Bioorthogonally Tunable Hydrogel Platform" Adv. Healthc. Mater., 2023, DOI:10.1002/adhm.202301701
107) Pol, M.; Gao, H.; Zhang, H.; George, O. J.; Fox, J. M.; Jia, X. "Dynamic Modulation of Matrix Adhesiveness Induces Epithelial–to–Mesenchymal Transition in Prostate Cancer Cells in 3D" Biomaterials, 2023, 299, 122180.
https://doi.org/10.1016/j.biomaterials.2023.122180.
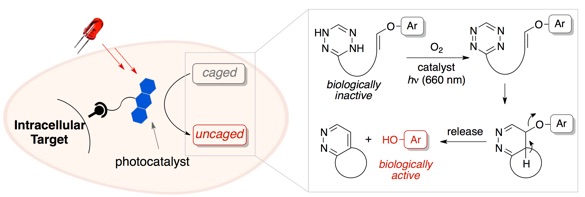
106) Rosenberger, J. E.; Xie, X.; Fang, Y.; Lyu, X.; Trout, W.; Dmitrenko, O.; Fox, J. M. Ligand-directed Photocatalysts and Far-red Light Enable Catalytic Bioorthogonal Uncaging inside Live Cells. J. Am. Chem. Soc. 2023, 145, 11, 6067–6078.
https://doi.org/10.1021/jacs.2c10655
105) Song, J.; Gao, H; Zhang, H.; George, O. J.; Hillman, A. S.; Fox, J. M.; Jia, X. "Matrix Adhesiveness Regulates Myofibroblast Differentiation from Vocal Fold Fibroblasts in a Bio–orthogonally Cross-linked Hydrogel" ACS Appl. Mater. Interfaces, 2022, 14, 51669–51682, https://doi.org/10.1021/acsami.2c13852
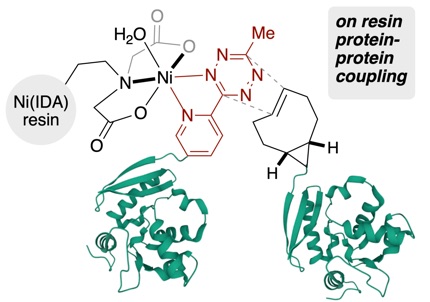
104) Scinto, S. L.*; Reagle, T.; Fox, J. M.* “Affinity Bioorthogonal Chemistry (ABC) Tags for Site-selective Conjugation, On-resin Protein-Protein Coupling, and Purification of Protein Conjugates” Angew. Chem. Int. Edit. 2022, 61, e202207661.
https://doi.org/10.1002/anie.202207661
103) George, O. J; Song, J.; Benson, J. M.; Fang, Y.; Zhang, H.; Burris, D. L.; Fox, J. M.*; Jia, X.* "Tunable Synthesis of Hydrogel Microfibers via Interfacial Tetrazine Ligation" Biomacromolecules, 2022, 23, 3017–3030. doi.org/10.1021/acs.biomac.2c00504.
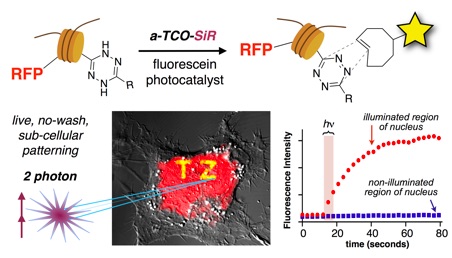
102) Jemas, A.; Xie, Y.; Pigga, J. E.; Caplan, J. L.; am Ende, C. W.; Fox, J. M. Catalytic Activation of Bioorthogonal Chemistry with Light (CABL) Enables Rapid, Spatiotemporally-controlled Labeling and No-Wash, Subcellular 3D-Patterning in Live Cells using Long Wavelength Light. J. Am. Chem. Soc. 2022, 144, 1647–1662. https://doi.org/10.1021/jacs.1c10390
Highlighted in C&E News: https://t.co/hNT3VDZzqC
Highlighted as a JACS Spotlight: https://pubs.acs.org/doi/10.1021/jacs.2c01203
Highlighted in Nature Chemical Biology: https://doi.org/10.1038/s41589-022-00987-8
Highlighted in Synfacts: DOI: 10.1055/s-0041-1738359
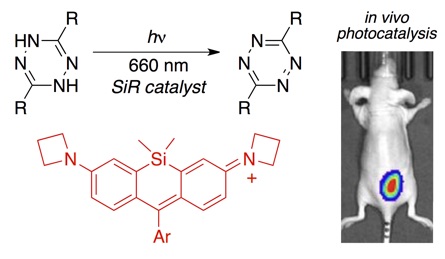
101) Wang, C.; Zhang, H.; Zhang, T.; Zou, X.; Wang, H.; Rosenberger, J.; Vannam, R.; Trout, W.; Grimm, J. B.; Lavis, L. D.; Thorpe, C.; Jia, X.; Li, Z.; Fox, J.M.* Enabling in vivo Photocatalytic Activation of Rapid Bioorthogonal Chemistry by Repurposing Si-Rhodamine Fluorophores as Cytocompatible Far-Red Photocatalysts, J. Am. Chem. Soc. 2021, 143, 28, 10793–10803. https://doi.org/10.1021/jacs.1c05547
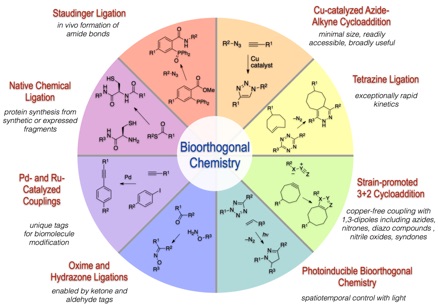
100) Scinto, S.; Bilodeau, D. A.; Hincapie, R.; Lee, W.; Nguyen, S. S.; Xu, M.; am Ende, C. W.; Finn, M. G.; Lang, K. Lin, Q.; Pezacki, J. P.; Prescher, J. A.; Robillard, M. S.; Fox, J. M.* Bioorthogonal Chemistry, Nature Reviews Methods Primers, 2021, 1, Article number: 30. https://doi.org/10.1038/s43586-021-00028-z
99) Pigga, J. E.; Rosenberger, J.; Jemas, A.; Boyd, S.; Dmitrenko, O.; Xie, Y.; Fox, J.. General, Divergent Platform for Diastereoselective Synthesis of trans-Cyclooctenes with High Reactivity and Favorable Physiochemical Properties, Angew. Chem. Int. Edit. 2021, 60, 14975-14980. https://doi.org/10.1002/anie.202101483
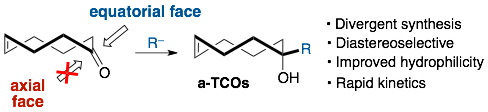
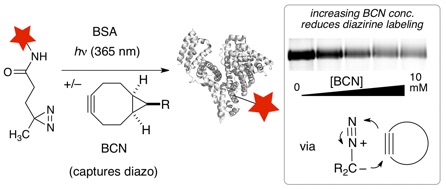
98) O’Brien, J. G. K., Jemas, A., Asare-Okai, P. N., am Ende, C. W.; Fox, J. M.* Probing the Mechanism of Photoaffinity Labeling by Dialkyldiazirines through Bioorthogonal Capture of Diazoalkanes. Org. Lett. 2020, 22, 9415-9420, doi:10.1021/acs.orglett.0c02714.
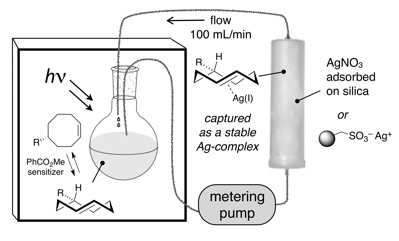
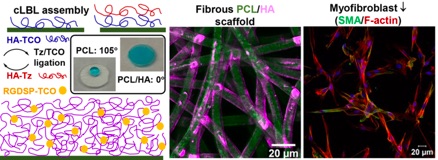
97) Ravikrishnan, A.; Zhang, H.; Fox, J. M.*; Jia, X.* Core–Shell Microfibers via Bioorthogonal Layer-by-Layer Assembly. ACS Macro Lett. 2020, 9, 1369–1375. https://doi.org/10.1021/acsmacrolett.0c00515
-
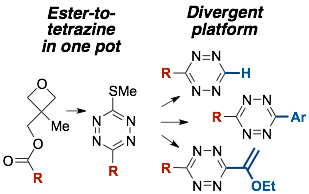
96)Xie, Y.; Fang, Y.; Huang, Z.; Tallon, A; am Ende, C.; Fox, J.M. Divergent Synthesis of Monosubstituted and Unsymmetrical 3,6‐Disubstituted Tetrazines from Carboxylic Ester Precursors. Angew. Chem. Int. Ed. 2020, 59, 16967-16973, doi:10.1002/anie.202005569
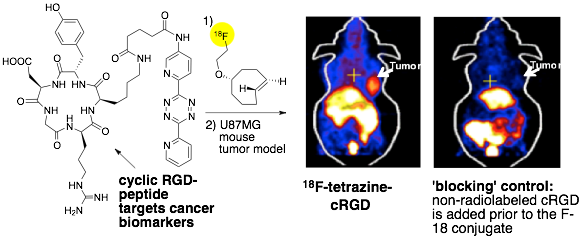
95) Feng, H.; Zhang, H.; Wang, M.; Vannam, R.; Wang, H.; Yan, X.; Ouyang, W.; Jia, X.; Fox, J. M.*; Li, Z.* Improving Tumor-to-Background Contrast Through Hydrophilic Tetrazines: The Construction of 18F Labeled PET Agents Targeting Non-Small Cell Lung Carcinoma Chem. Eur. J. 2020, 26, 4690-4694. https://doi.org/10.1002/chem.202000028
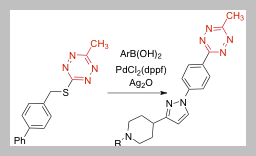
94)Lambert, W.; Fang, Y.; Mahapatra, S.; Huang, Z.; am Ende, C. W.; Fox, J. M. Installation of Minimal Tetrazines Through Silver-mediated Liebeskind-Srogl Coupling with Arylboronic Acids J. Am. Chem. Soc. 2019, 141, 17068-17074. https://doi.org/10.1021/jacs.9b08677
-
93)Pigga, J. E.; Fox, J. M. Flow Photochemical Syntheses of trans-Cyclooctenes and trans-Cycloheptenes Driven by Metal Complexation Isr. J. Chem. 2019, 60, 207-218.
https://doi.org/10.1002/ijch.201900085
Invited Review Article in a special issue honoring Professor Stephen L. Buchwald and John Hartwig on the occasion of the 2019 Wolf Prize
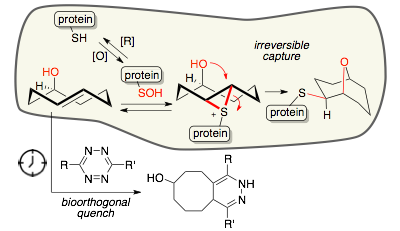
92) Scinto, S. L.; Ekanayake, O.; Sereviratne, U.; Pigga, J. E.; Boyd, S. J.; Taylor, M. T.; Liu, J.; am Ende, C. W.; Rozovsky, S.*; Fox, J. M.* Dual-Reactivity trans-Cyclooctenol Probes for Sulfenylation in Live Cells Enable Temporal Control via Bioorthogonal Quenching. J. Am. Chem. Soc. 2019, 141, 10932-10937. https://doi.org/10.1021/jacs.9b01164
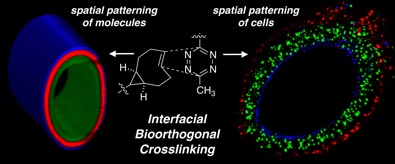
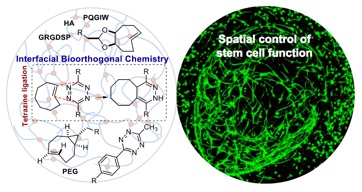
91) Dicker, K. T.; Moore, A. C.; Garabedian, N. T.; Zhang, H.; Scinto, S. L.; Akins, R. E.; Burris, D. L.; Fox, J. M.*; Jia, X.* Spatial Patterning of Molecular Cues and Vascular Cells in Fully Integrated Hydrogel Channels via Interfacial Bioorthogonal Crosslinking. ACS Appl. Mater. Interfaces, 2019, 11, 16402-16411. DOI: 10.1021/acsami.9b04383
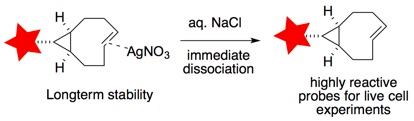
90) Fang, Y.; Judkins, J. C.; Boyd, S. J.; am Ende, C. W.; Rohlfing, K.; Huang, Z.; Xie, Y.; Johnson, D. S.; Fox, J. M. Studies on the Stability and Stabilization of trans-Cyclooctenes through Radical Inhibition and Silver (I) Metal Complexation Tetrahedron, 2019, 75, 4307-4317. https://doi.org/10.1016/j.tet.2019.05.038
Invited Contribution honoring Professor Stephen L. Buchwald on the occasion of the 2018 Tetrahedron Prize
89) Wang, M.; Vannam, R.; Lambert, W.; Xie, Y.; Wang, H.; Giglio, B.; Ma, X.; Wu, Z.; Fox, J. M.; Li, Z. Hydrophilic 18F-labeled trans-5-oxocene (oxoTCO) for efficient construction of PET agents with improved tumor-to-background ratios in neurotensin receptor (NTR) imaging. Chemical Communications 2019, 55, 2485-2488, DOI: 10.1039/C8CC09747J
88) Liu, S.; Moore, A. C.; Zerdoum, A. B.; Zhang, H.; Scinto, S. L.; Zhang, H.; Gong, L.; Burris, D. L.; Rajasekaran, A. K.; Fox, J. M.; Jia, X. Cellular Interactions with Hydrogel Microfibers Synthesized via Interfacial Tetrazine Ligation. Biomaterials 2018. 180, 24-35. https://doi.org/10.1016/j.biomaterials.2018.06.042
87) Hao, Y.; Song, J.; Ravikrishnan, A.; Dicker, K. T.; Fowler, E. W.; Zerdoum, A. B.; Li, Y.; Zhang, H.; Rajasekaran, A. K.; Fox, J. M.; Jia, X. Rapid Bioorthogonal Chemistry Enables in Situ Modulation of Stem Cell Behavior in 3D without External Triggers. ACS Appl. Mater. Interfaces 2018, 10, 26016–26027 DOI: 10.1021/acsami.8b07632
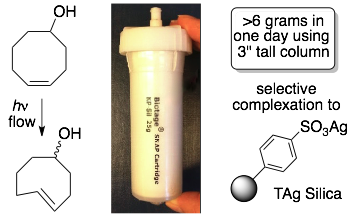
86) Darko, A.; Boyd, S. J.; Fox, J. M. Large Scale Flow Photochemical Syntheses of Functionalized trans-Cyclooctenes Using Sulfonated Silica Gel. Synthesis 2018 50, 4875-4882.
85) Dicker, K. T.; Song, J.; Moore, A.; Zhang, H.; Li, Y.; Burris, D. L.; Jia, X.; Fox, J. M. Core Shell Patterning of Synthetic Hydrogels via Interfacial Bioorthogonal Chemistry for Spatial Control of Stem Cell Behavior Chemical Science 2018, 9, 5394-5404 DOI: 10.1039/C8SC00495A.
84) Smith, N.; Sawicki, L.; Rohlfing, K.; Kharkar, P. M.; Boyd, S. B.; Kloxin, A.; Fox, J. Fast, Irreversible Modification of Cysteines through Strain Releasing Conjugate Additions of Cyclopropenyl Ketones. Org. Biomol. Chem. 2018. 16, 2164-2169 . DOI: 10.1039/C8OB00166A

83) Fang, Y.; Zhang, H.; Huang, Z.; Scinto, L.; Yang, J. C.; am Ende, C. W.; Dmitrenko, O.; Johnson, D.; S.; Fox, J. M. Photochemical Syntheses, Transformations, and Bioorthogonal Chemistry of trans-Cycloheptene and Sila trans-Cycloheptene Ag(I) Complexes. Chemical Science 2018, 9, 1953 - 1963. DOI: 10.1039/C7SC04773H

82) O’Brien, J. G. K.; Chintala, S. R.; Fox, J. M. Stereoselective Synthesis of Bicyclo[6.1.0]nonene Precursors of the Bioorthogonal Reagents s-TCO and BCN J. Org. Chem. 2018, 83, 7500–7503. DOI: 10.1021/acs.joc.7b02329.
Invited to Special Issue on 'Organic & Biocompatible Transformations in Aqueous Media'
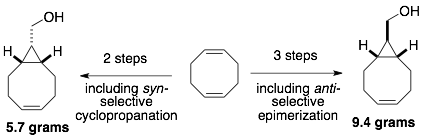
81) Lambert, W. D.; Scinto, L.; Dmitrenko, O.; Boyd, S. J.; Magboo, R.; Mehl, R. A.; Chin*, J. W.; Fox*, J. M.; Wallace*, S. Computationally guided discovery of a reactive, hydrophilic trans-5-oxocene dienophile for bioorthogonal labeling Org. Biomol. Chem. 2017, 15, 6640-6644. doi: 10.1039/C7OB01707C
80) Lamb, K. N., Squitieri, R. A., Chintala, S. R., Kwong, A. J., Balmond, E. I., Soldi, C., Dmytrenko, O., Reis, M. C., Chung, R., Addison, J. B., Fettinger, J. C., Hein, J. C., Tantillo, D. J.; Fox, J. M.*; Shaw, J. T.* Synthesis of Benzodihydrofurans by Asymmetric C-H Insertion Reactions of Donor/Donor Carbenoids. Chem. Eur. J. 2017, 23, 11843-11855. doi:10.1002/chem.201701630
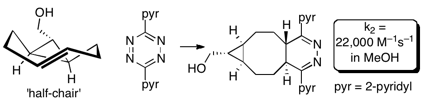
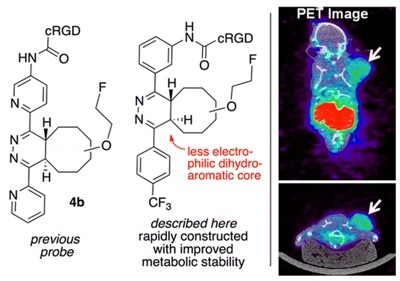
79) Zhang, H.; Trout, W. S.; Liu, S.; Andrade, G. A; Hudson, D. A.; Scinto, S. L.; Dicker, K. T.; Li, Y.; Lazouski, N.; Rosenthal, J.; Thorpe, C.; Jia, X.; Fox, J. M. Rapid Bioorthogonal Chemistry Turn-on through Enzymatic or Long Wavelength Photocatalytic Activation of Tetrazine Ligation J. Am. Chem. Soc. 2016, 138, pp 5978–5983. DOI: 10.1021/jacs.6b02168
* Highlighted in Chemical and Engineering News in their News of the Week section
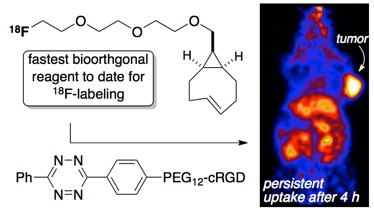
78) Wang, M.; Svatunek, D.; Rohlfing, K.; Liu, Y.; Wang, H.; Giglio, B.; Yuan, H.; Wu, Z.; Li, Z., Fox, J. M. Conformationally Strained trans-Cyclooctene (sTCO) Enables the Rapid Construction of 18F-PET Probes via Tetrazine Ligation Theranostics 2016; 6, 887-895. doi:10.7150/thno.14742
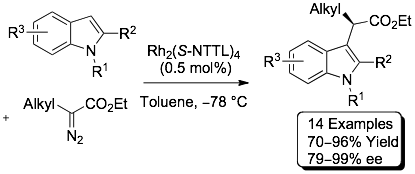
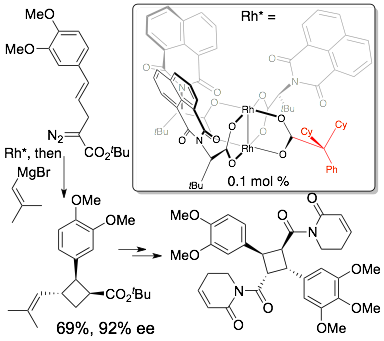
77) Panish, R. A.; Chintala, S. R.; Fox, J. M. Mixed-Ligand Chiral Rhodium(II) Catalyst Enables the Enantioselective Total Synthesis of Piperarborenine B 2016 Angew. Chem. Int. Ed. 2016, 55, 4983-4987. DOI: 10.1002/anie.201600766
*Highlighted in Synfacts: DOI: 10.1055/s-0035-1562120
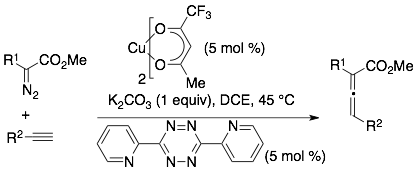
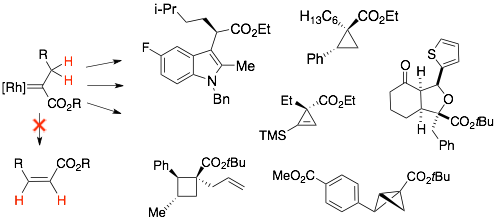
76) DeAngelis, A.; Panish, R.; Fox, J. M. Rh-Catalyzed Intermolecular Reactions of α-Alkyl-α-Diazo Carbonyl Compounds with Selectivity over β-Hydride Migration Acc. Chem. Res., 2016, 49, 115–127. DOI: 10.1021/acs.accounts.5b00425
-
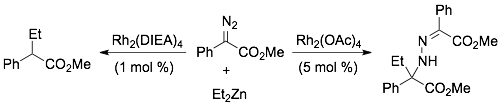
75)Panish, R.; Selvaraj, R.; Fox, J. M. Rh(II)-Catalyzed Reactions of Diazoesters with
Organozinc Reagents. Org. Lett. 2015, 17, 3978–3981. http://dx.doi.org/10.1021/acs.orglett.5b01836
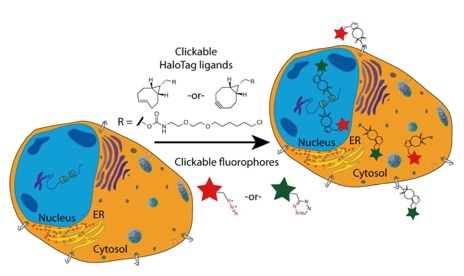
74) Murrey, H. E.; Judkins, J. C.; am Ende, C. W.; Ballard, T. E.; Fang, Y.; Riccardi, K.; Di, L.; Guilmette, E. R.; Schwartz, J. W.; Fox, J. M.; Johnson, D. S. Systematic Evaluation of Bioorthogonal Reactions in Live Cells with Clickable HaloTag Ligands: Implications for Intracellular Imaging. J. Am. Chem. Soc. 2015, 137, 11461–11475. http://dx.doi.org/10.1021/jacs.5b06847
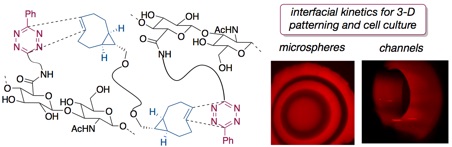
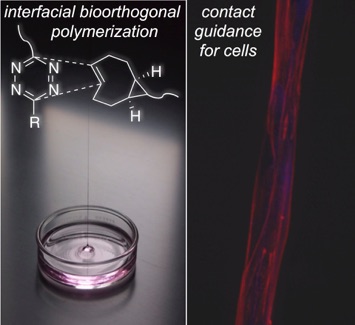
73) Liu, S.; Zhang, H; Remy R. A.; Deng, F.; Mackay, M. E.; Fox, J. M.*; Jia, X.* Meter–Long Multiblock Copolymer Microfibers via Interfacial Bioorthogonal Polymerization Adv Mater, 2015, 27, 2783-90. DOI: 10.1002/adma.201500360.
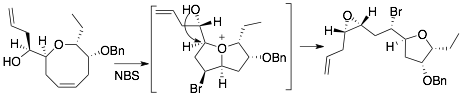
72) Taylor, M. T.; Fox, J. M. , Biosynthesis of the C15-acetogenin laurepoxide may involve bromine-induced skeletal rearrangement of a Δ4-oxocene precursor, Tetrahedron Lett., 2015, 56, 3560–3563. doi:10.1016/j.tetlet.2015.03.009
~Invited article in special issue dedicated to Howard Zimmerman
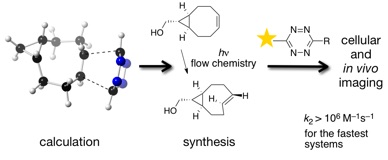
71) Selvaraj, R.; Giglio, B.; Liu, S.; Wang, H.; Wang, M.; Yuan, H.; Chintala, S. R.; Yap, L.-P.; Conti, P. S.; Fox, J. M.*; Li, Z.* Improved Metabolic Stability for 18F PET Probes Rapidly Constructed via Tetrazine trans-Cyclooctene Ligation Bioconjugate Chem. 2015, 26, 435–442. DOI: 10.1021/acs.bioconjchem.5b00089
70) Selvaraj, R.; Chintala, S. R.; Taylor, M. T.; Fox, J. M. Org. Synth. 3-Hydroxymethyl-3-phenylcyclopropene. 2014, 91, 322-337. DOI: 10.15227/orgsyn.091.0322
69) Darko, A.; Wallace, S.; Dmitrenko, O.; Machovina, M. M.; Mehl, R. A.; Chin, J. W.; Fox, J. M. Conformationally strained trans-cyclooctene with improved stability and excellent reactivity in tetrazine ligation Chemical Science 2014, 5, 3770. DOI: 10.1039/C4SC01348D
68)Zhang, H.; Dicker, K. T.; Xu, X.; Jia, X.; Fox, J. M. Interfacial Bioorthogonal Cross-Linking ACS Macro Letters 2014, 3, 727. http://pubs.acs.org/doi/abs/10.1021/mz5002993
-
67)Fox, J. M.; Robillard, M. In vivo chemistry - pushing the envelope Curr. Opin. Chem. Biol. 2014, 21, v-viii. http://www.sciencedirect.com/science/article/pii/S1367593114000982
66) Selvaraj, R.; Fox, J. M. An efficient and mild oxidant for the synthesis of s-tetrazines Tetrahedron Lett. 2014, 55, 4795–4797. doi:10.1016/j.tetlet.2014.07.012
65) Selvaraj, R.; Fox, J. M. trans-Cyclooctene- a stable, voracious dienophile for bioorthogonal labeling Curr. Opin. Chem. Biol. 2013, 17, 753-760. http://dx.doi.org/10.1016/j.cbpa.2013.07.031
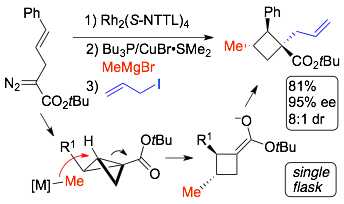
64) Panish, R.; Chintala, S. R.; Boruta, D. T.; Fang, Y.; Taylor, M. T.; Fox, J. M., Enantioselective Synthesis of Cyclobutanes via Sequential Rh-catalyzed Bicyclobutanation/Cu-catalyzed Homoconjugate Addition. J. Am. Chem. Soc. 2013, 135, 9283-9286. DOI: 10.1021/ja403811t
63) Xie, X.; Fox, J. M., Diastereoselective Syntheses of Highly Substituted Methylenecyclopropanes via Copper- or Iron-Catalyzed Reactions of 1,2-Disubstituted 3-(Hydroxymethyl)cyclopropenes with Grignard Reagents. Synthesis 2013, 1807-14. DOI: 10.1055/s-0033-1338876
Invited article to a special issue dedicated to Scott Denmark
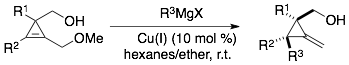
62) Xie, X.; Fox, J.; Li, Y., Selective Syntheses of Δα,β and Δβ,γ Butenolides from Allylic Cyclopropenecarboxylates via Tandem Ring Expansion/[3,3]-Sigmatropic Rearrangements. Org. Lett. 2013, 15, 1500–1503 http://dx.doi.org/10.1021/ol400264a.

55) Hassink, M. D.; Fox, J. M. Functionalized Cyclopropenes and Methylenecyclopropenes from Dianions of 3-Hydroxymethylcyclopropenes Synthesis, 2012, 44, 2843-2850. NIHMS387488, PMC3128944.
Invited contribution to Special Topic Issue on the synthesis and chemistry of small rings
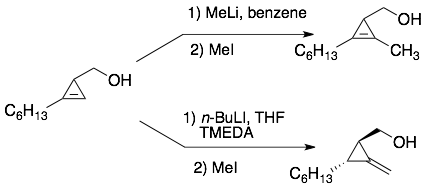
Invited contribution in special issue in honor of Carolyn Bertozzi, Tetrahedron Young Investigator Award
-
44)Dong, Z.; Plampin, J. N. III; Yap, G. P. A.; Fox, J. M. ‘Minimalist Endgroups for Control of Absolute Helicity in Salen- and Salophen-based Metallofoldamers’ Org. Lett. 2010, 12, 4002–4005. DOI: 10.1021/ol101583v
-
43)DeAngelis, A.; Boruta, D. T.; Lubin, J.-B.; Plampin, J. N. III, Fox, J. M. “The Chiral Crown Conformation in Paddlewheel Complexes” Chemical Communications 2010, 4541-4543.
42) Xie, X.; Yang, Z.; Fox, J. M. “Stereospecific synthesis of alkylidenecyclopropanes via sequential cyclopropene carbomagnesation/1,3-carbon shift" J. Org. Chem. 2010, 75, 3847–3850.

-
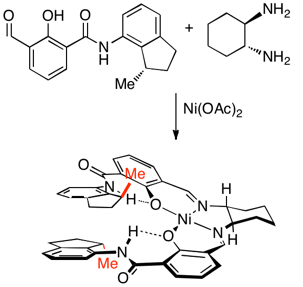
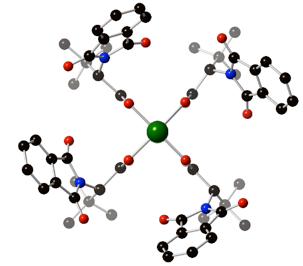
41)Fisher, L. A.; Zhang, F.; Yap, G. P. A.; Fox, J. M. “Weak absolute helicity direction in Ni–salen by trans-cyclohexane-(1R,2R)-diamine” Inorg. Chim. Acta. 2010, 364, 259-260.
The Fox Group Designing New Reactions for Chemical Biology
New Reactions for Biology
Applications
Synthetic Methods