Background & Goals of Project
This project involves a collaboration with the group of Joel Schneider in the Department of Chemistry & Biochemistry at UD. Bone remodeling is a process that occurs at the surface of healthy bone. It involves many biological components whose precise functions are unknown. Recently, the crystal structure was solved for osteocalcin (Hoang, et al. Nature 2003 425, 977-980), a protein that binds to the mineral components of bone and is known to exhibit chemotactic activity.
Predictions can be made as to how osteocalcin binds to hydroxyapatite, a common model/surface material for bone. We have explored the possibility of using rational design to develop a peptide that will bind to bone by mimicking the structure of the key subunits of osteocalcin. Osteocalcin contains an alpha helical segment with three glutamic acid residues that undergo vitamin-K-dependent post-translational carboxylation. All three carboxylated glutamic acid residues are positioned on the same solvent-exposed face of the protein and are believed to play a role in the binding of osteocalcin to bone.
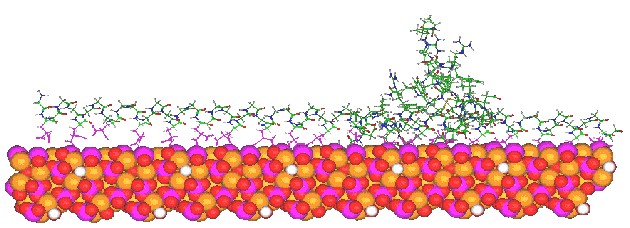
The theorized structure of osteocalcin (OC) bound to the surface of hydroxyapatite. OC appears to the right, while a 50-residue peptide is superimposed over the Gla-containing helix of OC to demonstrate that longer helicies may have the same binding capabilities as the short Gla-containing helix in osteocalcin.
Skills You Will Develop on this Project
Students and postdocs involved in this project will be exposed to standard and advanced protein synthesis and purification methodologies, high-resolution AFM measurements, surface instrumental analysis techniques such as XPS and TOF-SIMS, and the development of new surface chemistries.
Examples of Publications from the Group