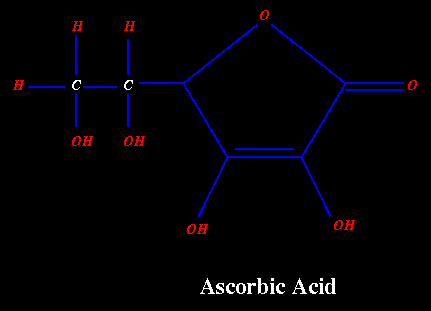
STRUCTURE OF ASCORBIC ACID
The structure of ascorbic acid was determined by E.L.Hirst, A.Szent-Gyorgyi, F.Michael and K.Kraft in 1933. Ascorbic acid is a 2,3-enediol-L-gulonic acid-g -lactone. L-ascorbic acid, L-xyloascorbic acid and L-threo-hex-2-enomic acid g -lactone are the chemical names recognized in 1965 by the IUPAC-IUB commission on Biochemical Nomenclature (49) and Chemical Abstracts for vitamin C.

SYNTHESIS
In 1934, T.Reichstein and A.Grussner published a method of synthesis which is still used for the technical production of ascorbic acid. The most important steps are: catalytic hydrogenation of D-glucose to D-sorbitol; oxidation of D-sorbitol to L-sorbose; production of diacetone L-sorbose and its oxidation with permanganate in alkaline solution to the carbonic acid; hydrolytic cleavage of acetone to form 2-oxo-L-gulonic acid; and enolixation to ascorbic acid.
REACTIONS
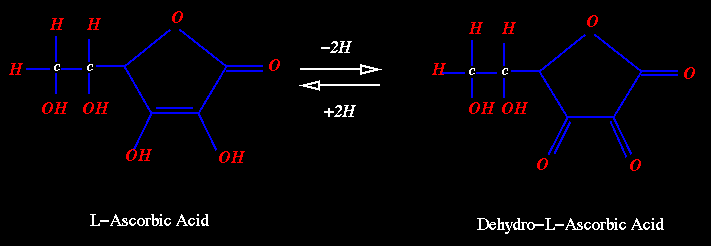
Oxidation
of ascorbic acid
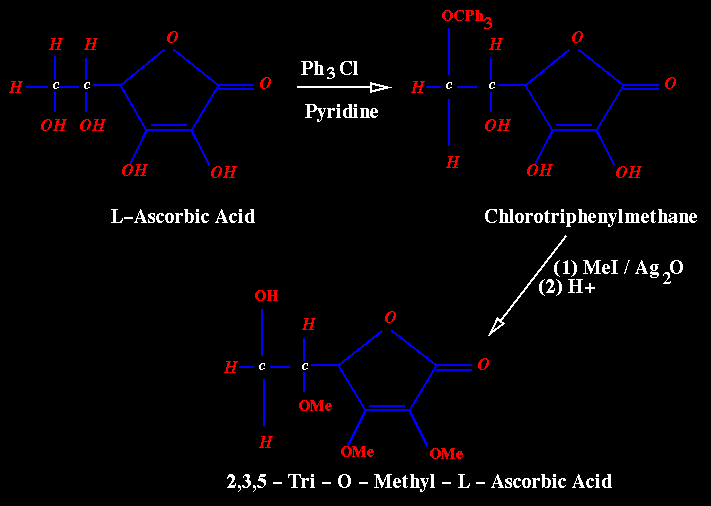
The primary alcohol group at C-6 undergoes ready triphenylmethylation (tritylation) with chlorotriphenylmethane and pyridine.
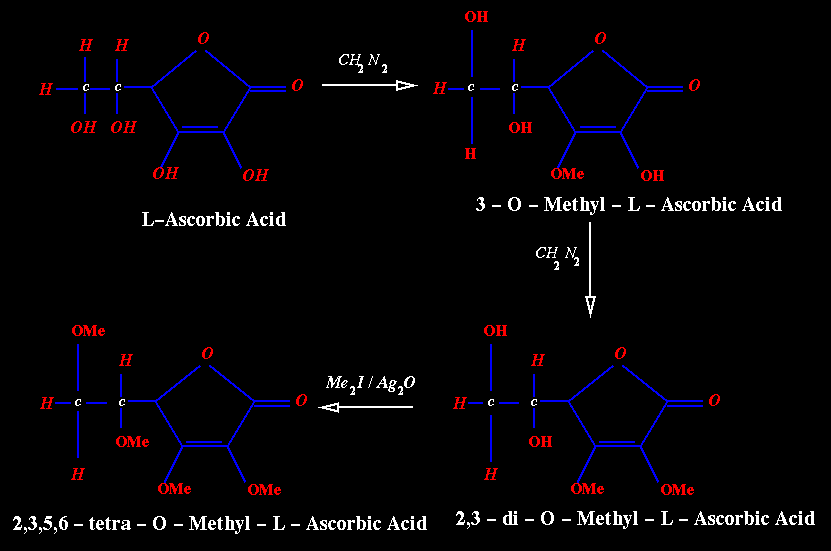
Methylation:
Methylation
of L-ascorbic acid with diazomethane has given explanation as to the tautomeric
nature of the vitamin.