St. John's Wort

St. John's Wort, a popular
herbal remedy for depression, grows as a yellow flower, Hypericum
perforatum, in nature. It is also used to treat anxiety and sleep
disorders, and is commonly sold in drug and health food stores.
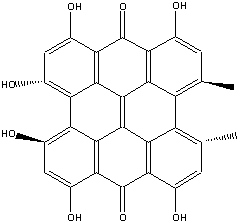
The active ingredient in St. John's Wort is hypericin, an intricate structure
of polycyclic phenols, which looks like this:
Hypericin
St. John's Wort is popular among
mildly depressed patients because it is an over the counter remedy, which
is cheaper than prescription drugs, and it has fewer side affects, such
as nausea, headache, diarrhea, and impaired sexual function, which
is common with prescription antidepressants.
Although the exact mechanism for
how the body uses St. John's Wort is not known, there have been several
proposed. One idea that is popular is that it works as a re-uptake
inhibitor. In the brain, there are neurotransmitters which help the
brain communicate with the body. One is serotonin, which is the transmitter
in the brain responsible for communication among nerve cells, as well as
the chemical in the brain which cause people to feel happy. The idea
is that St. John's Wort inhibits the re-uptake of this chemical in the
brain, causing it to be more abundant. The presence of this chemical
makes people happy and reduces depression. This mechanism is also
responsible for the function of the illegal drug ecstasy. The other
theory behind the functioning of St. John's Wort has to do with the immune
system. In this system, the substance IL-6 is responsible for the
communication between cells, and leads to an increase in adrenal regulatory
hormones, a culprit of depression. St. John's Wort may reduce the
levels of this hormone to decrease depression.

St. John's Wort, however, is not
free of side affects. It can often cause dry mouth, dizziness, gastrointestinal
symptoms, sensitivity to sunlight, and fatigue. It can also have
serious affects on newly developed cells, as noted by a study done at Loma
Linda University, where St. John's Wort was found to "damage reproductive
cells" at high concentrations in hamster oocytes. This is a warning
that pregnant women, or those trying to get pregnant should not take this
drug.
As well as these symptoms, St. John's
Wort is also known to react with prescription drugs, which is why a physician
should be consulted before use. One such example found by the National
Institutes of Health is with the drug, Crixivan (indinavir), which is a
protease inhibitor used to treat HIV. St. John's Wort was shown to
interfere with the pathway used to metabolize this drug, by speeding it
up, which in turn decreases the blood concentrations of all PI's used to
treat HIV. Speeding up this pathway used by many prescription drugs,
such as for heart conditions, seizures, depression, birth control, and
anti-rejection of new transplants (cyclosporine), can cause major health
problems by reducing their effects.
As well as the drug interactions,
there have been questions about the usefulness of St. John's Wort for mild
to moderate depression. According to a survey done in the May 2000
issue of Consumer Reports, the top method of treatment was prescription
drugs. The next most effective treatments were exercise, diet, and
meditation, with St. John's Wort being deemed to help little, if at all.
This is strong evidence to support that lifestyle changes are a healthier,
more effective, less dangerous way to combat mild to moderate depression.
This report can be found at http://www.consumerreports.com
.
Another study done by the LA Times
demonstrates a question of quality control. Does the quantity of
St. John's Wort listed on the bottle actually exist in each pill?
This study shows that the answer to that question
is no.

This study shows not only the dangerous
variation from label to pill, but also that the American public is being
ripped off. First of all, this is dangerous because the pills that
have a higher concentration than the label says could be cause people to
overdose. The pills that contain less than what the label says show
that some Americans are spending money on products where 80% of it is missing.
That is like buying an entire box of cereal, and not having enough to fill
up a bowl for breakfast.
Because of these dangerous drug
interactions, lack of quality control, and side effects, both known and
unknown, the best policy that the FDA could take would be to ban advertising
them. They cannot prevent the drugs from being on the market, and
they cannot recall them without suspicion that the herbs are harmful, but
the FDA can limit the public's exposure, as well as reduce false advertising.
It is often this persuasive advertising which is responsible for the confusion
of the drug's purposes, limits, side effects, and interactions. By
eliminating the advertising, the public is more likely to seek physician's
assistance instead of trusting the media and false label claims.
BACK TO MAIN