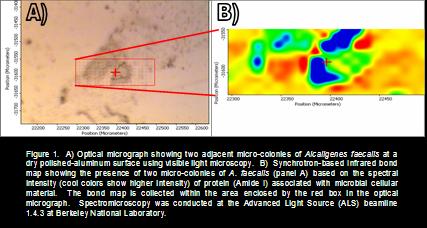
|
18th World Congress of Soil Science
July 9-15, 2006 - Philadelphia, Pennsylvania, USA |
 |
Mineralogically- and biologically-assisted oxidation of arsenic (As) was investigated using both high-temporal (millisecond to second) and high-spatial (3 - 10 μM) resolution infrared (IR) spectroscopic techniques. In natural systems, arsenic speciation is impacted by redox chemistry occurring at the surface of minerals, particularly manganese oxides, and microorganisms, e.g. Alcaligenes faecalis. Establishing the redox state of As in the environment, as well as the reaction kinetics that govern redox transformations, is critical for understanding toxicity and mobility. Rapid scan attenuated total reflectance (ATR-IR) spectroscopy and synchrotron-based IR spectromicroscopy were utilized to establish the degree of influence that these reactive interfaces have on arsenic speciation by first exploring the reaction kinetics in homogeneous systems followed by heterogeneous systems achieving high-temporal resolution. Spectromicroscopy was then used to establish the spatial distribution of arsenic reactivity in heterogeneous systems to isolate the influence of individual phases, i.e., minerals versus microorganisms (e.g., Figure 1). The results from spectroscopic investigations will be coupled with concurrent research using batch soil chemical techniques to generate a comprehensive understanding of As dynamics in contaminated soil systems.