| Instructor: David L. Kirchman (kirchman@udel.edu)
September 23, 1997
MAST 634
Lecture 6: Thermodynamics
Important Points From Last Lecture
Energy Flow in Phycobilin - containing algae
Some algae only have PC and APC Absorption and fluorescence characteristics
Direction correct, but energy transferred as excitons, not fluorescence Phycobilin proteins in phycobilisome Another example of energy transfer -- lack there of
Heterotrophy
General comments about what to learn
How is ATP produced: catabolism First, what is ATP?
What's this mean?
See page 51 of V&V
In general: A + B = C + D
delta G1
What's important is delta G3 <O, not whether delta G1 or delta G2 <O How is ATP made? Two general classes of reactions
In oxidative phosphorylation (= "respiration")
Glycolysis and Kreb cycle
 Summary of Substrate - level (SL) phosphorylation and oxidative phosphorylation
(OP)
Bucher 1897 "life in test tube" ---> argument against "vital force", separate from chemistry What e- acceptor?
e-acceptor: H+ + O2 + 2e- ---> H2O How is organic matter catabolized?
What's a large molecule?
Large compounds ------------------> monomers,
True even for higher organisms
What happens next depends on compound and whether O2 is present
See diagram Let's focus on glucose degradation What happens if O2 not present
Anaerobic respiration: e - acceptor other than O2 (next lecture) Fermentation: oxidation of NADH Most "common" fermentation -- > in your text book Name of fermentation ---> endproduct released
LDH
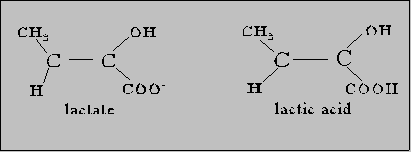 Lactate dehydrogenase (LDH) "old" enzyme ---> used in phylogenetic studies Occurs in muscles, but soreness and muscle fatigue due not to lactic
acid but other acids.
Usually, lactate excreted and converted back to glucose in liver ---> in mammals, but not necessary in all organisms Effect of switching from aerobic growth to fermentation in yeast Observations
How to explain these observations?
How to determine efficiency?
Consider a general reaction
At equilibrium, i.e. No net change
Products
[C]c [D]d
When not at equilibrium
What's delta Go?
free energy of formation
Additional problem STD biochem conditions Delta G' & delta Go' [H2O]
= 1 ; not [H2O] = 55.5M
Example delta Go' for
Delta Go = (2* - 526.6)- -917.2
(page 51 on delta Go----> delta Go' i.e. taking into account H+) What's efficiency? Lactate fermentation ---> 2 ATP's delta Gfo' for
ATP = -30.5, so 2 ATP, total E harvested is 61 kJ
In comparison:
Many types of fermentation Note: Problem with all fermentation is acid production Drawbacks of fermentation
Strategies of invertebrates ( and others) to deal with anoxia Many invertebrates can survive long period without O2
|