| Instructor: Dr. David L. Kirchman (kirchman@udel.edu)
September 16, 1997 MAST 634 Lecture 4: Light Reactions Important Points from Last Week
Photosynthesis: Light reactions
What does a plant need to fix CO2?
Remember: in chemoautotrophy, oxidization of reduced inorganic compounds produced e- for NAD(P)H and created gradients for ATP synthesis We will try to answer the following questions
General structure of P.S. apparatus answers these questions
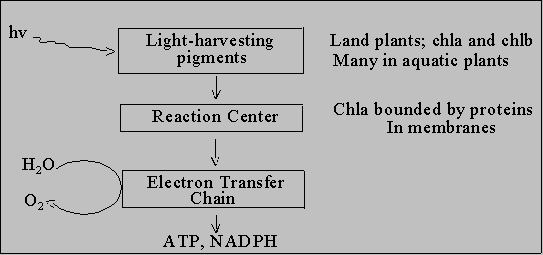 What happens when light hits a pigment? It's now "electronically excited". Energy (E) has to go somewhere. 1. Heat
2. Fluorescence
What is wave length?
Einstein is a mole of photons So, E excited > E emitted
small amount (3-6%) of total absorbed light goes off as fluorescence = in vivo fluorescence very important in examining plants by remote sensing, growth rates 3. Exciton transfer; resonance energy hv pigment A ---> pigment B
Let's discuss reaction center and electron transfer chain. Next lecture
will deal with light harvesting pigments.
[A side: Goal here is to understand final "answer", not just give it.] Known since Priestly in 1772 that plants evolve O2 But where does O2 come from? Modern day experiment use stable isotope of O, 18O
C.B. van Niel at Hopkins Marine Lab (Stanford)
light
green photosynethic bacteria: example of anoxygenic photosynthesis only bacteria do this eukaryotic plants and cyanobacteria: evolve O2 = oxygenic photosynthesis van Niel deduced that O2 come from splitting of H2O (oxidation of H2O) Anoxygenic P.S. bacteria cannot split O2 Why not?
deltaEo´ = Eo´pigment - Eo´H2A acceptor donor since deltaEo´>O for deltaG<O
Specifically for H2O Eo´pigment > 0.82 Another redox problem
Eo´ V
Here: Delta Eo´ = Eo´NADP/NADPH-
Eo´pigment
[NOTE: This web program cannot transcribe some greek symbols; this is why "delta" or other characters may be written out] Various "solutions" to this problem during evolution of photosynthesis Purple bacteria: Cyclic e- transport version of this in oxygenic
photosynthesis
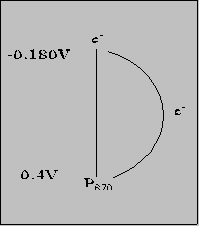 P870 = not strong enough oxidizer for H2O P870 = type of bacterial chlorophyll (bchl) Px Maximum absorbance at x nm Net Result
Where do these bacteria get e- for NAD(P)H?
Green Bacteria 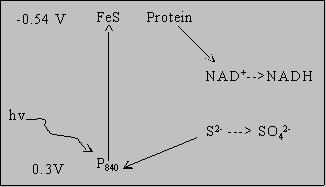 FeS protein strong enough reducer for NADH synthesis P840 strong enough oxidizer for S2- oxidation but not water Finally, oxygenic photosynthesis: see handout Three components in "Z scheme" 1. Photosystem II: H2O --->
1/2 O2 + proton gradient
Some evidence for this scheme: action of DCMU
Flow of e-
Balance between PSI and PSII
ATP synthesis
Proton-motive force, once again
|