| Instructor: Dr. David L. Kirchman (kirchman@udel.edu)
September 18, 1997 Important points from last lecture
Redox reactions: pigments and electron donors Z scheme of oxygenic photosynthesis (PS) Arrangement of 3 components of PS in membranes 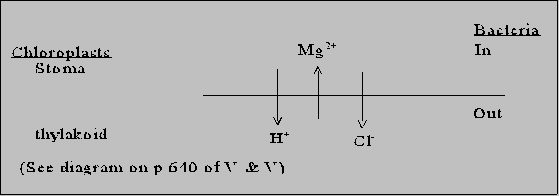 H+ balanced by Mg+2 and Cl-. End result is no gradient in charge. Unlike oxidative phosphorylative, PMF solely due to proton gradient. Also, direction H+ pump opposite to what mitochondria do. But otherwise, ATP synthase very similar. Balance between PSI and PSII Depends on NO3-
or NH4+ as N source
In words: NO3- needs to be reduced to oxidation state of NH4+ by NAD(P)H. A more complete answer to: What determines balance between PSI and PSII? [From R. Geider] The balance between in vivo PSI and PSII reaction rates should be related to relative demands for NADPH (which requires linear electron flow and involves both PSII and PSI for transfer of electrons from H2O to NADPH) versus ATP (which can be generated by either non-cyclic photophosphorylation involving proton transfer associated with donation of electrons from PSII to the cytochrome b6/f complex [together with release of H+ from water splitting] or cyclic photophosphorylation involving donation of electrons to the b6/f complex from PSI). What, you may ask, determines the relative requirements for NADPH and ATP? Well, biochemistry determines the relative demands. This includes biochemical composition of cells, ion transport across the cell membrane, nitrate vs ammonium as N source, operation (or not) of a CO2 concentrating mechanism. Of course, it is not that simple, because the ratio of H+ transfer through the ATP synthetase to ATP synthesis is expected to vary, and respiration may play a role in providing reductant (NADH) and/or ATP even under illuminated conditions. The Mehler reaction (PSI mediated electron transfer to oxygen) can also lead to pseudocyclic photophosphorylation. The Mehler reaction requires that oxygen first be liberated at PSII before it can subsequently be consumed at PSI. The Mehler reaction is not insignificant, and may equal 50% of gross oxygen evolution in cyanobacteria at light saturation. A good text is D. Lawlor's new revised (1996?) edition with a title something like "Photosynthesis: mechanisms metabolism and molecular biology." Of course, it is directed at terrestrial vascular plants rather than aquatic photosynthesis. Use of fluorescence in marine biology and oceanography A. Chl concentrations
Excitation
Spectrofluorometer: wavelengths can be changed  much more sensitive and easier than spectrophotometric methods Chl a approximate
measure of algal carbon = 50* [chla]
Photosynthetic rate proportional to 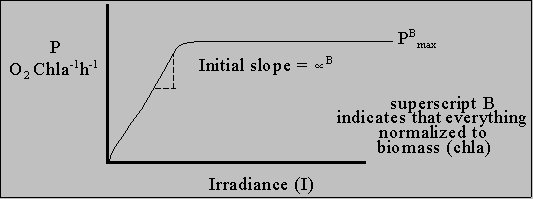 at low light: P =
In words: P = (light intensity)*(area absorbing light)*(PS activity per area per mole of photons) Light harvesting by aquatic plants Special problem with living in water
Iz = Io e-zk
where
Three major solutions: "accessory" pigments
General comments on structure of pigments
How is light energy transferred to reaction centers (RC)?
One example: Marine cyanobacteria Cyanobacteria = blue-green algae
Important marine cyanobacteria
Light harvesting pigments in Synechococcus allophycocyanin
(APC)
phycobilin: light absorbing part = chromosphore What is sequence of energy transfer, eventually to chl a?
Marine Synechococcus has PC, APC, but lots of PE Pure cultures are blood red Distribution of pigments in different organisms
Absorption and fluorescence characteristics
this is direction by which energy is transferred but it's not by fluorescence (too slow), but by excitons. Phycobiliproteins in complex: phycobilisome "Structure follows function".
|