
Puffer fish (Fugu rubripes) |

Dendrobates granuliferus |
Hooded Pitohui April 1993, National Geographic. |
CASE STUDY IN MOLECULAR EVOLUTION NO. 1
Written by Harold B. White
CHEM-647 BIOCHEMICAL EVOLUTION, FALL 2002

Puffer fish (Fugu rubripes) |

Dendrobates granuliferus |
Hooded Pitohui April 1993, National Geographic. |
Long before evolutionists knew much about proteins and nucleic acids, they recognized the value of small molecules for chemotaxonomy and for inferring phylogenetic relationships (1,2). They expected that organisms related by divergence from a common ancestor would produce related molecules, i.e. shared characteristics define certain clades. The distribution of many small molecules supports this logic. For example, all vertebrates use phosphocreatine as an energy source during short bursts of activity while various groups of invertebrates use other phosphagens such as phosphoarginine (1,3). On the other hand, after studying bacteria, fungi, and plants, Nes and Nes (4) concluded that the distribution of various lipid molecules does not conform to the usual interpretations of phylogeny. They found similar lipids in distantly related organisms. What evolutionary processes could explain the unexpected presence of similar or identical molecules in distantly related organisms?
It may seem likely that the lengthy metabolic pathways required to synthesize particular terpenes, steroids, alkaloids, pigments and other small biological molecules would evolve only once and be retained by divergent evolution from a common ancestor. However, that assumption neglects the potential for convergent evolution given the strong selective pressure to produce a limited number of functionally important molecules and the fact that selection can act over million of years on countless organisms. A careful study of biosynthetic pathways in different organisms reveals that ubiquitous molecules like NAD (5) and lysine (6) can be made in different ways. These and other examples suggest that dissimilar organisms can possess identical small molecules as the result of convergent evolution.
The evolutionary process leading to a complex metabolic pathway probably occurs in stages and takes time. However, a biosynthetic capacity can be lost with a single mutation that even may have a selective advantage (7). The distant ancestors we share with other metazoans appear to have lost many biosynthetic pathways possessed by their unicellular ancestors (8) as a consequence of diets rich in vitamins and amino acids. Such selective losses can further confound the evolutionary interpretation of the distribution of small molecules even among related organisms. Conversely, selective gains via horizontal gene transfer or plasmid aquisition offer ways for organisms to acquire traits that do not come from immediate ancestors.
The fact that most plants contain chlorophyll and most eukaryotes contain cardiolipin highlights yet another evolutionary phenomenon affecting the distribution of small molecules--endosymbiosis (9,10). A great deal of evidence supports the hypothesis that chloroplasts and mitochondria evolved from free living microorganisms that merged their respective metabolic abilities with a large host cell in an intracellular symbiotic relationship more than two billion years ago. These organelles now are integral parts of eukaryotic cells but contain small molecules that belie their prokaryotic origin. There are examples of other endosymbioses that are restricted to a limited range of hosts yielding animals that can photosynthesize (11) or live on H2S, O2, and CO2 (12).
In some cases the distribution of small molecules indicates "you are what you eat." Various species of milkweed (Asclepius spp.) produce distasteful toxic cardiac glycosides and thereby protect themselves from many herbivores (13). Several species of insect have independently (convergently) evolved resistance to these compounds and now feed preferentially on milkweed. Among these is the well known monarch (milkweed) butterfly (Danaus plexippus) (14) that harmlessly incorporates cardiac glycosides into its own body (15,16). While natural selection normally favors forms and colors that conceal (17-19), chemically protected animals (e.g. skunks) benefit from advertizing. These aposematic organisms include the monarch butterflies and other insects that feed on milkweed and share its toxins. Predators quickly learn to recognize and avoid these brightly colored insects (20). Other organisms such as the viceroy butterfly, Limenitis archippus (21), that do not feed on milkweed and may or may not contain other toxic compounds (22) mimic the monarch butterfly and escape predation.
While the transfer of certain cardiac glycosides produced by the milkweed to other organisms through feeding is well understood, the scattered occurrence of other toxins among marine and terrestrial vertebrates around the world cannot be explained so easily. In Japan the pufferfish, Spharoides rubripes, is prized food that must be prepared by licensed cooks who know how to remove the poison-containing ovaries, liver, and skin. Nevertheless each year many people die from eating improperly prepared fugu. The poison is tetrodotoxin, a potent neurotoxin that blocks ion channels (24,25). Anthropologists believe that tetrodotoxin in pufferfish from Haiti is actually used to produce zombies (26). Tetrodotoxin also occurs in an unrelated fish, Gobius crininger, in eggs and embryos of the Pacific Coast salamander, Taricha torosa (24), in the venom of an Australian octopus, Hapalochlaena maculosa (27), and in poison-dart frogs of the genus Atelopus (28). Curiously, a chemically unrelated but potent neurotoxin, homobatrachotoxin, is found in other aposematic Central American poison-dart frogs (29) and this same compound has recently been isolated from the feathers and skin of the Pitohui, a brightly colored poisonous bird from New Guinea (31-33). These animals don't look like each other and don't live near each other, yet they contain the same toxic compound. The structures of tetrodotoxin and homobatrachotoxin are given below.
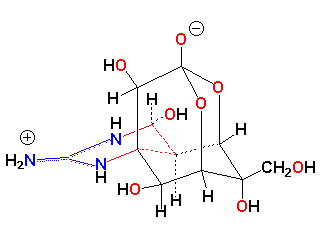 |
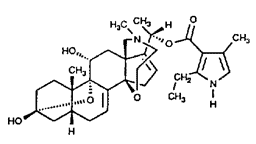 |
|
|
|
The most recent reference below was published in 1993. Thus, part of your effort will be to search the more recent literature using PubMed, the Web of Science, or some other search engine. Developing the ability to efficiently track down articles in the primary literature is an important skill for people in the sciences.
NOTE: Many years ago before photocopiers were generally
available in the library, some student in this class tore out all or parts
of some of the articles on the reference list below. They thereby deprived
all subsequent students of these resources. Such behavior is reprehensible
and is grounds for expulsion.