Problem Set No. 4
Reactions
Involving HMG CoA
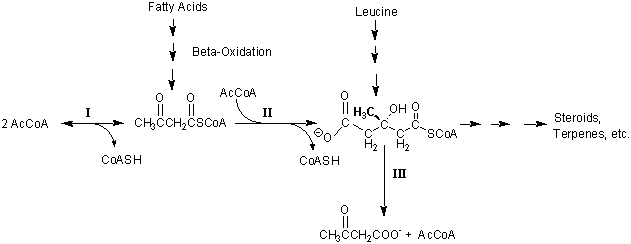
3-Hydroxy-3-methyl glutaryl CoA (HMG CoA), is an intermediate in leucine degradation, steroid and terpene biosynthesis, and ketogenesis as is diagrammed below. The following questions relate to the chemistry and biochemistry of reactions involving HMG-CoA.

1. (3 Points) Citrate cleavage enzyme (ATP: citrate lyase) is very strongly inhibited by the nonphysiological compound, (-) hydroxycitrate [(2S, 3S)-2,3 dihydroxy-3-carboxyl-glutarate] (Arch. Biochem. Biophys. (1969) 135, 209-217), a fact that generated interest in it as a drug to lower fat synthesis by Hoffmann-LaRoche in the 1970's. Both fatty acid synthesis and steroid biosynthesis are inhibited by (-) hydroxycitrate (Arch. Biochem. Biophys. (1972) 150, 183-190). What does this imply about the intracellular location and precursors of steroid synthesis? As with fatty acid biosynthesis (Problem Set 3, Question 3), one can measure cholesterol synthesis from 2-14C-pyruvate and by measuring the incorporation of 3H from 3H2O. For fatty acid synthesis, it was observed that when unlabeled citrate was added to the system, the amount of 14C-labeled fatty acid formed decreased while the amount of 3H-fatty acid formed increased under the same conditions. Would the incorporation of label from 2-14C-pyruvate and 3H2O look the same as a function of increasing citrate concentration if incorporation of 3H and 14C into steroids were being measured? Explain. (Please note: You will not find an answer to this in a textbook. I am looking for a thoughtful analysis, comparison, and prediction.)
2. (1 Point) Ketogenesis (Reactions I, II & III) is not likely to be inhibited by (-) hydroxycitrate. Taken with the fact that there are isoenzymes for reactions 1 and II, (J. Biol.Chem. (1973) 248, 2275-2284) where in the cell does ketogenesis take place? How many intracellular pools of HMG CoA are there in a liver cell?
3. (3 Points) Using the chemical structures of the substrates and products, compare the reactions catalyzed by HMG CoA synthase (II), HMG CoA lyase (III); citrate synthase (TCA cycle enzyme), and citrate cleavage enzyme in terms of their similarities. [Note the similarities and significance of their similar Enzyme Commission (EC) numbers. Also, it will help to display these reactions so that the similarities and differences can be directly compared.] Predict where the equilibrium of each reaction would lie based on the differences in the reactions. (Remember that equilibrium is a thermodynamic state and has nothing to do with mechanism. Also, we are considering the reaction in isolation, e.g. a purified enzyme in a test tube, not coupled to other metabolic reactions in a cell.)
4. (3 Points) Enzymes, with extremely few exceptions, are stereospecific in their reactions. Thus, they can distinguish between two apparently identical (prochiral) groups on a single molecule. For example, citrate has a plane of symmetry, yet aconitase, ATP-citrate lyase, and citrate synthase can tell the difference between the two halves. While it may seem rather arcane, people interested in enzyme mechanism want to determine the precise stereospecificity for every enzyme. Thus, in the case of HMG CoA lyase, it becomes an intellectual challenge to distinguish which of two chemically identical hydrogen atoms is retained and which is removed. To this end, two samples of stereospecifically tritiated HMG CoA (A & B) were synthesized (Eur. J. Biochem. (1975) 53, 255-264); structures as shown below.
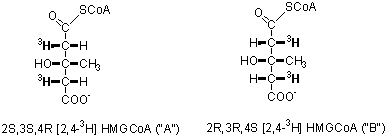
These compounds were used to determine the stereochemistry of the HMG CoA lyase reaction. The reaction of HMG CoA to AcCoA and acetoacetate was carried out in deuterated water (2H2O). The AcCoA with a chiral methyl group was converted in two enzymatic reactions (shown below) to fumarate and the amount of tritium retained was measured.
From HMG CoA sample A, 73% of the tritium was lost while only 23% of the tritium was lost from sample B. Based on these data, reconstruct the predominant stereochemistry of the AcCoA and malate derived from A & B. Does the HMG CoA reaction mechanism proceed with retention or inversion of configuration?
NOTES - Protium (1H) is preferentially but not exclusively lost in the malate synthase reaction. The condensation of AcCoA with glyoxyllate proceeds with inversion of configuration (Acct. Chem. Res. (1975) 8, 1-10). The fumarase reaction proceeds by a trans elimination of water (J.A.C.S. (1959) 81, 6333 & (1961) 83, 3634).
This assignment has several purposes:
1. To give you practice in thinking
about the chemical details of metabolic reactions.
2. To provide perspectives on
the interrelationships of pathways.
3. To illustrate how isotopes
are used experimentally.
4. To illustrate how enzymes
can be used as reagents.
5. To relate experimental data
to testable models.
This assignment will be evaluated on the following
elements:
1. Obtaining reasonable answers.
2. Clear and logical explanations.
3. Appropriate use of chemical
structures to illustrate important points.
4. Turned in on time.