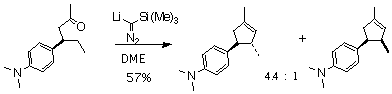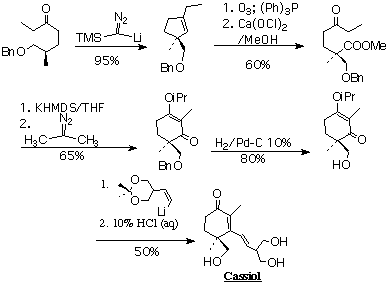
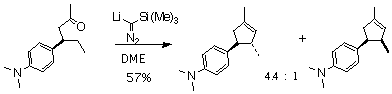
The core of my dissertation is the asymmetric synthesis of cassiol. This potent antiulcerant was isolated from chinese cinnamon, used since ancient times as a stomach remedy. This molecule consists of a cyclohexenone ring bearing a single center of asymmetry and an unsaturated side chain bearing a 1,3 propanediol moiety.
To effect the synthesis (Scheme 1), I have developed general methodology for forming symchiral cyclohexane derivatives. In support of this objective, I have developed the generation and subsequent C-H insertion reactions of alkylidene carbenes into a practical, high yielding method for the construction of functionalized cyclopentenes. Elaboration of the side chain led to the invention of a self chelating vinyl lithium reagent.
In a complimentary project, I investigated the diastereoselectivity of this cyclization. Indeed (Scheme 2), I was able to demonstrate that the C-H insertion reaction into a prochiral methylene proceeds with significant diastereocontrol.
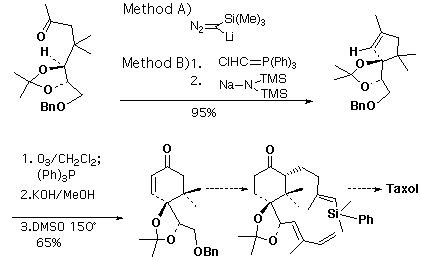
In addition, I applied my methods to the synthesis of a taxol A-ring synthon (Scheme 3). I originally cyclized the ketone (readily obtained in seven steps, starting with L-tartaric acid) using trimethylsilyldiazomethyllithium. I was able to supplement this route with a more economical alternative strategy for generating the alkylidene carbene: conversion of the ketone to the vinyl chloride followed by base promoted elimination to the carbene.
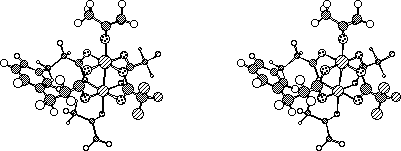
In earlier work at the University of Delaware, I synthesized the first dirhodium tetracarboxylate complex with two ligands bridged (Figure 1). This molecule was designed using Michael Zerner's INDO method and Molecular Mechanics. The x-ray structure that I obtained was nearly an exact match to the model, giving credence to our design methodology for asymmetric dirhodium catalysts.
My work in this research group has resulted in a total of eight publications.