
"
Home Page: Taber home page.
Chem 334
Department of Chemistry and Biochemistry
University of Delaware
A typical chromatography column is shown in Figure 7.2. The weight of silica gel ("flash", 40-60 mm) is usually about 20 to 30 times the amount of pure compound in the crude sample. Usually a column height of about 10 cm of silica gel is sufficient to effect separation. For larger-scale separations, a wider, not a longer, column is used. It is necessary to have some free volume in the tube above the adsorbent for a supply of solvent. A separatory funnel mounted above the column can be used as a convenient solvent reservoir.

Fig. 7.2 Preparation of a chromatography column. Note that the silica gel is packed into the column as a dry powder.
The column is prepared in the following manner:
1. With a long wire (a straightened coathanger works well), tamp a small wad of glass wool into the bottom of the column. Gently pour a layer of sand over the glass wool, remove the wire, and tap the column to settle the sand. The sand retains fine particles and also provides a flat horizontal base for the adsorbent column.
2. Add the weighed amount of dry silica gel is added in a fine stream. Tap the tube gently to dislodge air bubbles and to level the top of the silica gel.
3. Dissolve your sample in a volatile solvent - methylene chloride works well. Add to the resulting solution an amount of silica gel that is one-tenth of the weight of silica gel that you are using in your column. Evaporate the solvent - the rotary evaporator works well. Add the resulting dry powder to the top of the silica gel in your column, and tap the settle this new layer.
4. Add a layer of sand (both the top and the bottom sand layers should be about 0.5 cm thick) to prevent disturbance of the surface when solvent is added. Tap again to settle the new layer.
Choosing a solvent:
Find a solvent combination that moves your desired product with an Rf of about 0.3 on TLC. This same solvent mixture should give good separation on the column. For a very close separation of a mixture of two or more components, gradient elution is often effective. If, for instance, the solvent mixture that gives an Rf of about 0.3 is 20% MTBE / petroleum ether, one might elute the column with first 5% MTBE / petroleum ether, then 10% MTBE / petroleum ether, then 20% MTBE / petroleum ether.
To elute the column:
1. Add solvent, gently at first, so as to not disturb the sand on top of the column. When the solvent starts to drip out of the bottom of the column, begin collecting fractions - test tubes in a test tube rack work well. Keep adding solvent to the top of the column so it does not go dry. If the column runs slowly, gentle air pressure (5 ~ 15 psig) may be applied.
2. Check each fraction by thin layer chromatography (TLC). Note that more than one fraction can be spotted on a plate. Combine in a tared flask the fractions that contain only pure product, then evaporate the solvent.
EXPERIMENTS
SAFETY NOTE
Care should be taken with the volatile solvents used. Diethyl ether, methyl tert-butyl ether, and petroleum ether are flammable. Ferrocene and acetylferrocene are toxic.
A. SEPARATION OF FERROCENE AND ACETYLFERROCENE (Microscale)
Acetylferrocene is prepared from ferrocene (Fig. 7.3) by a Friedel-Crafts reaction, and the product is readily separated from starting material by column chromatography. Since both materials are colored, it is easy to follow their separation.
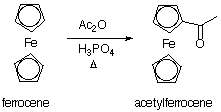
Fig. 7.3 Preparation of acetylferrocene.
PROCEDURE
Preparation: Combine 0.4 g of ferrocene, 2 mL of acetic anhydride and 0.5 mL of 85% phophoric acid in a test tube. Stir the mixture well, then heat it in a 60 to 80 oC water bath for 5 minutes. Add an equal volume of crushed ice, stir until the ice has melted, then extract the aqueous suspension with petroleum ether. Dry the solution over anhydrous K2CO3, and decant the dried solution into a clean flask. Check this solution by TLC, using 4:1 petroeum ether / MTBE. You should observe an orange spot for the product at Rf = 0.31, and perhaps a yellow spot for the starting material at Rf = 0.79. Add 0.5 gram of silica gel to the rest of the solution, and evaporate to give a dry orange powder.
Purification: Prepare a silica gel column as outlined above, using five grams of silica gel in a 1.9 cm i.d. x 30 cm column. Tap to settle the silica gel, then add the dry orange powder prepared above. Tap to settle, then add a layer of sand. Have fifteen test tubes, each of which can hold at least 5 mL, ready in a test tube rack.
Add 75 mL of 4:1 petroeum ether / MTBE in portions to the column. As the solvent moves down the column, you will probably observe a yellow band followed by an orange band. Collect the effluent from the column in 5 mL fractions, and check each of the fractions by TLC (remember, you can often spot three or even four fractions on the same TLC plate). Combine in a tared flask the fractions that have the pure product spot, evaporate the solvent, and record the weight of the product acetylferrocene. Record the melting point and calculate the yield of the reaction. The product may be crystallized by scratching with a little petroleum ether or hexane. The literature m.p.' s of ferrocene and acetylferrocene are 173-174 oC and 85-86 oC respectively.