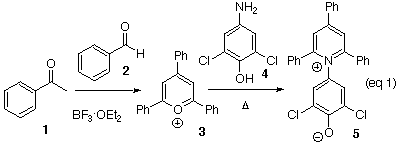
Preparation of ET(33):
A Solvatochromatic Dye
Solvent polarity is an elusive concept not easily determined solely by consideration of a chemical structure. Solvatochromatic dyes, represented by ET (33) 5,1 are a set of tools that have been developed to assess solvent polarity. These dyes show absorption maxima that vary linearly 2 with the dielectric constant
e and the refractive index nD of the solvent.We could envision a range of undergraduate laboratory experiments centered around the solvatochromatic dyes. Unfortunately, the first3,4 such dye reported in this journal required a 24-hour reflux in its preparation. This is not desirable in most undergraduate teaching labs. An attempt to remedy this5 used as a starting material 4-amino-2,6-diphenylphenol, which is no longer commercially available. The preparation of this substance reported by the authors was not reproducible either in their hands or in ours.
While the best2 of the solvatochromatic dyes requires facilities for its synthesis that are not available in most undergraduate teaching laboratories, a very acceptable dye, 5, is in fact easily prepared. The synthesis of the salt requires two steps. Acetophenone and benzaldehyde are first heated in the presence of boron trifluoride etherate to form the 2,4,6-triphenylpyrillium tetrafluoroborate salt 3 (eq 1).6 This salt on heating with 2,6-dichloro-4-aminophenol (4)1 gives ET(33) (5) as a dark brown salt, sufficiently pure for further use.

Suggested Applications
a) A simple experiment can be performed by dissolving 5 in a series of solvents of decreasing polarity, and observing the colors that result. A solution in deionized water, for instance, is faintly yellow, indicating end absorption barely into the visible. A solution in 100% ethanol, on the other hand, is deep red, indicating absorbance well into the green. Solutions in a range of ethanol-water mixtures show a continuum of colors. Other solvents1 can be used also, of course.
b) A more advanced experiment, if a UV/VIS spectrophotometer is available (we have used both a Hewlett Packard 8452A UV/Vis Diode Array Spectrophotomer and a Perkin Elmer 553 Fast Scan UV/Vis Spectophotometer) is to have the students create a standard curve, plotting known ethanol concentrations in water against the precise
lmax (409 nm for pure water, 470 nm for pure ethanol) of the resulting dye solution. They should then be able to determine very closely the ethanol content of an ethanol/water unknown by dissolving a little of the dye in the unknown, and then measuring the lmax of the resulting solution. Our experience has been that undergraduate students take a keen interest in this ability to accurately measure unknown ethanol concentrations.
Experimental Section
Preparation of the pyrillium salt (3): Boron trifluoride etherate (6.5 mL, 24.5 mmol) was added dropwise over 1 min to a stirred solution of acetophenone (2.2 mL, 18.84 mmol) and benzaldehyde (0.96 mL, 9.42 mmol). The reaction flask was fitted with a short path condenser and a receiver to collect the ether vapors. The flask was heated to about 100°C (bath temperature) for 1.5 h. The reaction mixture was cooled, then taken up in 11 mL of acetone. Addition of 110 mL of diethyl ether to the reaction solution precipitated the golden yellow salt 3, which was collected by filtration (1.25 g, 34% yield). Determine the melting point.
Preparation of ET(33) (5): The 2,4,6-triphenylpyrillium tetrafluoroborate salt (3) (1.25 g, 3.15 mmol) and 2,6-dichloro-4-aminophenol (4) (0.98 g, 5.5 mmol) were dissolved in hot ethanol (95%, 15 mL). To this stirred solution was added sodium acetate (0.82 g, 10.0 mmol). The solution was heated to reflux for 2 h. The mixture was allowed to cool and 15 ml of 5% aqueous NaOH was added. The bulk of the ethanol was removed by distillation, precipitating the dark brown product 5, which was collected by filtration. The residue was washed with 1% aqueous NaOH and air dried to give 5 as a dark brown powder (1.21 g, 61% yield). Determine the melting point.
Literature Cited
(1) Kessler, M.A.; Wolfbeis, O.S. Chem. Phys. Lipids. 1989, 50, 51.
(2) Effenberger, F.; Wurthner, F. Angew. Chem. Int. Ed. (Eng). 1993, 32, 719.
(3) Minch, M.J.; Shah, S.S. J. Chem. Educ. 1977, 54, 709.
(4) Abdel-Kadler, M.H.; Steiner, U. J. Chem. Educ. 1983, 60, 709.
(5) (a) Osterby, B.R.; McKelvey, R.D. J. Chem. Educ. 1996, 73, 260. (b) The references for this article are found at J. Chem. Educ. 1997, 74, 900.
(6) Vogel, A.I. Practical Organic Chemistry, 5th ed.; Longman/Wiley: New York, 1989: p. 1169.