The Retort

Sulfonium Ions
I’ve glossed over sulfonium ions as alkylating agents in previous semesters, but because of their biological significance I’m discussing that section of Section 7.10 in the text.
Use in Biochemistry
The compound S-adenosylmethionine is used in nature to transfer methyl groups to a variety of nucleophiles. It is itself synthesized by the nucleophilic attack of the sulfur in methionine on a molecule of ATP (adenosine triphosphate):

The positively-charged sulfur atom of a sulfonium ion is a good leaving group. Attack of a nucleophile on the methyl group (the least sterically-hindered group on the sulfur) results in net transfer of the methyl group to the nucleophile via SN2.
Mustard Gas
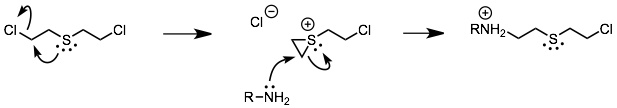
The sulfur mustards are thioethers with chloride leaving groups beta- to the sulfur. SN2 attack of the sulfur on the beta-carbon produces an episulfonium ion, which is a good alkylating agent. Formation of the episulfonium ion is similar to the formation of epoxides shown in Fig. 7.109 in the text.
A variety of nucleophiles, including amino groups on DNA, can then be alkylated by these episulfonium ions. The massive destruction the sulfur mustards wreak inside a victim cause severe, agonizing, blistering burns.
Monday, November 12, 2012