The Retort

Resolution of Enantiomers via Diastereomeric Salt Formation: Naproxen
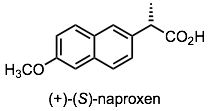
Racemic naproxen is resolved by a modification of the salt-formation technique we learned in class. There are two twists to the protocol that make the process even more useful for commercial production of enantiomerically pure naproxen. First, in a twist known as the Pope-Peachy method, only half an equivalent of chiral amine is used. Half an equivalent of a cheaper, optically inactive amine base is used in its place. This not only makes the process cheaper, but results in a more dramatic difference in solubilities. The conjugate base of the desired (d)-(+)-enantiomer crystallizes out with the conjugate acid of the chiral amine, with high selectivity. This leaves the more soluble salt product of (l)-(-)-naproxen and the achiral amine behind in solution.

The second twist is that, by simply heating the “mother liquor” (the solution that remains after filtration) , the undesired enantiomer of naproxen can be converted back to a racemate. You will learn in CHE322 that protons next to a carbonyl are modestly acidic; this means that if you have a methine stereocenter next to a carbonyl you always have to be wary that its configuration can get “scrambled” by deprotonation/reprotonation. In this case, however, we want the configuration to equilibrate back to a racemic mixture, and the achiral base that was used, plus heat, is enough to do the job.
This process allows naproxen to be synthesized by a simpler, cheaper route as a racemate instead of a single enantiomer. Not only can the two enantiomers be resolved by this process, but the “undesired” enantiomer is converted to the desired, rather than being discarded--effectively doubling the yield of product.
Reference: Harrington, P.J.; Lodewijk, E. “Twenty Years of Naproxen Technology”. Organic Process Research and Development, 1997, 1, 72-76.
Monday, October 8, 2012