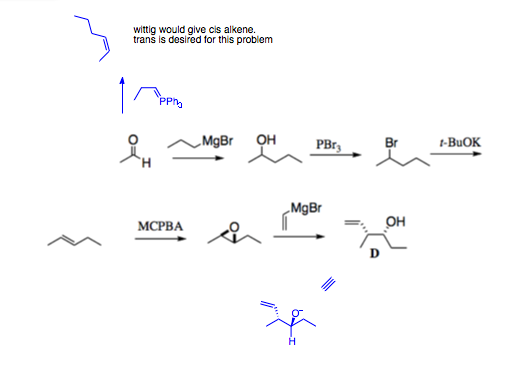
Answer to student question
Saturday, November 19, 2011
Q:
I have a question from Taber's 2006 homework 9, question #3. I was
wondering if it were possible to form the alkene using PPh3 and the
aldehyde instead of the way he completed it. Also, why does the O
group invert when the MgBr group adds to the epoxide?
A:
good questions. My additions to the Taber answer are in blue in the graphic
I think you are asking if it would be possible to use a Wittig reaction, instead of elimination of Br, to form the alkene. The issue is that you need to make the trans-alkene, and Wittig reaction gives the cis-alkene. mCPBA would be used to put in the epoxide.
There was only inversion on the left side. I redrew the structure (see pasted picture) to show that it is simply a bond rotation, and not inversion, on the right side.
-JMF