
 Acid Rain Can Be Prevented Without Compromising Industral Ouput
Acid Rain Can Be Prevented Without Compromising Industral Ouput

 Acid Rain Can Be Prevented Without Compromising Industral Ouput
Acid Rain Can Be Prevented Without Compromising Industral Ouput
Although the combustion of fossil fuels is necessary to provide the power
that drives our society and industries, it also presents the issue of acid
rain. Acid rain is one of the severest consequences that our society faces
due to engaging in heavy industry. Industrial processes relying on fossil
fuels for power have led to an increase in acid rain due to greater
emissions of gases such as sulfur dioxides (SOx) and nitrogen oxides
(NOx). In the atmosphere these gases react with water to form
acids which cause a downward shift in the pH of the atmospheric water.
This water eventually condenses and falls to the ground as acid rain,
where it has profound effects on the environment and its inhabitants.
The technology exists for the prevention of acid rain with respect to industrial
emissions, yet there is a strong debate over whether the implementation of
such technology is beneficial to the industrial entities. Evidence
indicates that employing various techniques to eliminate acid rain can
ultimately benefit industry and society.
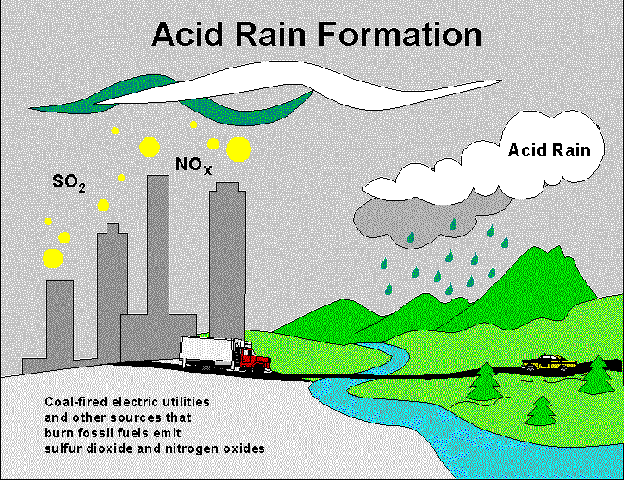
A common
misconception about SO2 emissions is that cars and transportion are
the primary sources. It is important to note that over 93% of all sulfur
dioxide emissions are industral based (with only 3.7% transport based). However,
cars and other forms of transportion (trucks, airplanes, boats, etc.) are
responsible for large amounts of NOx being released into the atmosphere
( creating smog and ozone pollution ).
While some of their effects are similar, the large scale release of these
two componds represent two different major
enviornmental problems and should not be confused.
After SO2 and NO2 are released into the
atmosphere they are not particularuly acidic or soluble in H2O.
However they are converted
to H2SO4 and HNO3 in the atmosphere
(using cloud condensation nuclei as catalases1) which
are both acidic and soluble in H2O. The process by which this
occurs is :
(1)
(2)
the hydroperoxyl radical then produces more hydroxyl radicals via the reaction :
(3)

The sulfuric acid produced in equation (2) is then released from the atmosphere in rain water. A simlar
process occurs to produce HNO3 and rid it from the atmosphere. The
lifetime of SO2 is only a few days and as a result rainfall containing
high amounts of H2SO4 only occurs a few hundred miles
from the source of emission. This effect can be seen in a recent
pH map of the United States where the lowest pH readings are centered
around the NorthEast (the country's industral backbone). Another important fact
pertaining to this map is that rainfall is naturally acidic. Carbon dioxide found
in the atmosphere is dissolved into the water droplets existing in clouds and
effectly lowers the pH of rain to about 5.7 log units. Despite carbon dioxide's
role in lowering the pH of rainfall, large increases in the amounts
of carbon dioxide found in the
atmosphere are not expected to cause a large decrease in the pH of rainfall. ( For
instance, a doubling in CO2 concerntrations results in only about a
0.2 unit decrese in pH)1.
The SO2 released be power plants can by reduced by appoximately 90% through the use of
a scrubber or more offically known as flue gas desulfuriztion. This is achieved through the use of calcium carbonate.
(4)
(5)
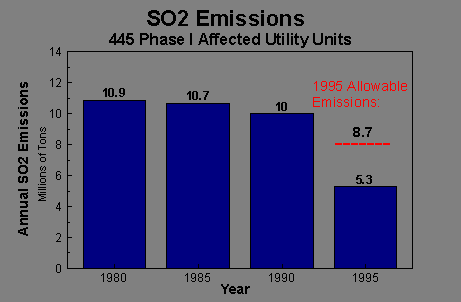
In order to reduce the effects of acid rain, the EPA has recently
initiated an Acid Rain Program
aimed at "significantly reducing electric utilties emissions
of sulfur dioxide and nitrogen oxides".
The
Clean Air Act's primary goal is reduction of annual
SO2
emissions by 10 million tons below 1980 levels (0.9 million tons).
Legislation introduced by the EPA calls for a "two-phase tightening of
the restrictions placed on fossil fuel-fired power plants."
Meeting the new emission restrictions has forced companies to use existing
techonoloies to reduce the level of emissions.
A large number of emission reducing processes are availiable such as
wet and dry flue gas desulfuriztion
,
dry acid gas scrubbing systems for solid
waste incineration
,
electrostatic precipitators
and
fabric filters
and are
currently being empolyed by companies like
General Electric.
The effectiveness of these
processes can be easily seen by the almost 50% reduction of the total
national amount of sulfer dioxide
released from the 1980 emission level (about 40% below the EPA allowable
emissions for 1995).
Despite the large decrease in SO2 emissions by 1995, the annual
heat input from utilities remained constant (even increasing in some states) as
can be shown by examining figures2
2
and
3.
This shows that current techonolgies are able to cause
significant reduction of
sulfur dioxide emissions without having an addverse effect on industral output.
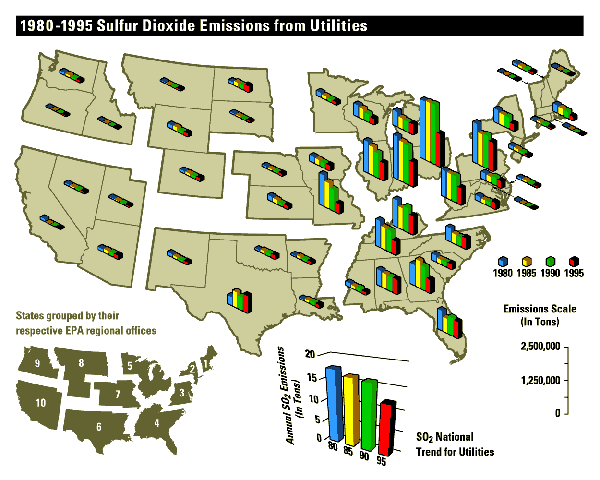 figure 2
figure 2
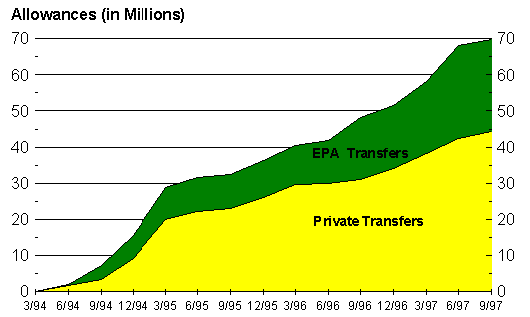
The EPA has devised an
Allowance Trading Plan
to help ease the cost of installing clean air
systems. Each year the allowable amount of emissions are are divided into credits and
given to companies where the emissions of each company is not to exceed thier number of
credits. Companies are allowed to sell their credits as they chose. By selling there credits
to a larger company, smaller companies are able to afford the costs of installation.
The volume of allowance trading shows that more and more companies are taking advantage
of the EPA's system and are updating their plants. Since the second phase of the EPA's plan will
increase the number of units involed from about 450 to 2000, this is a
positive indicator that the future goals (for the year 2000) will be able to
be met.
 Other companies are using other approaches to help curve the cost of reduced emissions.
Their solution, which is by far the best long term solution to pollution, is to recycle their
waste products into other marketable goods.
Other companies are using other approaches to help curve the cost of reduced emissions.
Their solution, which is by far the best long term solution to pollution, is to recycle their
waste products into other marketable goods.
There have been some companies that have taken advantage of the cleaning of
the waste produced by coal-powered electric plants. The Tennessee Valley
Authority's Cumberland Power plant is one such example. During the production
of power, the sulfur gases produced are sent through electrostatic
precipitators, which remove the fly ash, and then continue through the flue gas
desulfurization scrubbers where the gas is sprayed with a scubber slurry that
absorbs the SO2. This SO2 laden acidic liquid is then neutralized by the
addition of crushed limestone which results in calcium sulfite. With a heavy
blast of air, the material is oxidized and forms calcium sulfate- more commonly
known as "gypsum." This Plant produced over a million tons of gypsum annually.
In the past, this product was shipped off to a disposal site where it was
buried at a cost to the company and could possibly harm the environment.
At the Cumberland Plant, they have signed several contracts with other
companies, SYNMAT and Temple Inland, for the purchase of the high quality
gypsum. These companies would then use the gypsum to produce wallboard,
cement additives, and for use in loosening soil for agricultural purposes.
The wallboard plant was built next to the power plant to minimize hauling
distances (reduce pollution from transport vehicles). Not only does this
process make more jobs available, but it also reduces environmental pollutants
while generating another source of income for the company. Still other companies such as
ReUse
use the collected sulfer to make soil feritzlers. This will pay for
the extra costs involved with the installation of the advanced technologies.

New techonologies are being developed to create more efficeint filtering systems.
Companies are developing methods using
activated coke/carbon
to help reduce toxic gas emissions (claims a 98% sulfur removal rate).
Other researchers3 report
that vast improvements in the reaction rate of calcuim with SO2 are possible by reducing the
diameter of the calcium particles used in scubbers (thus increase their effectivness).
NOx gasses have had recent solutions discovered. Ballard Power systems has
designed a fuel cell that runs on external fuel. This process converts
hydrogen and oxygen into water and produces electricity. (diagram)
The only drawback to implementing this system is the cost. The fuel cells
cost roughly ten times the amount of the internal combustion engine, running
the price from near $3000 to $30,000. The fuel cells are hoped to be in cars
within the next decade.