|
Environmental Effects
|
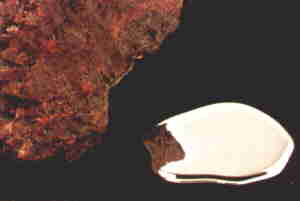 |
Mercury
exists in three forms in the environment: elemental
mercury, organic mercury, and inorganic mercury.
In the environment, mercury may be converted from one form to another
through oxidation/reduction and
methylation/demethylation reactions.
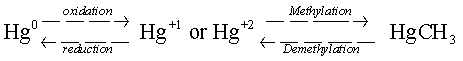
- The global contribution of
mercury emissions to the atmosphere from man made sources is 2,000 to 4,000
tons per year (tpy). The amount
from natural resources is similar.
- 87% of the anthropogenic
contribution is from combustion (waste and fossil fuels).
- 33% of the U.S.
contribution is from utilities.
- The EPA identified
electric utilities as the largest remaining non-environmental source of
mercury into the air
- Water accumulation is
emphasized because consumption of water-based organisms is the main way
humans are exposed to mercury released by utility plants.
- In1998, 84% of the
deposition in Lake Michigan was atmospheric.
- Methylated mercuries are
the most important form of mercury when dealing with toxicity and exposure.
o
Hg2+ contributes the most to methylmercury.
It is highly soluble in water and so is easily transported from the
atmosphere by rain.
o
Nearly all mercury that accumulates in fish is methylmercury.
o
Rain can contain as much as 30x the EPA’s level of safe mercury.
- Concentrations in water
bodies greatly vary depending on the region of the U.S., proximity to local
sources, weather, characteristics of the water and associated organisms,
season, wind pattern, and the geography of surrounding land.
- A 1 yr. study of Lake
Champlain found that half of total amount of mercury exported to
connecting streams and rivers for that year happened in one day.
This was due to a peak snow melt.
- Various studies of
mercury concentrations in fish and water have found that levels greatly
differ among sites.
- Anthropogenic deposition
in the North East is from:
- 47%
local
- 30% outside
- 23% global reservoir
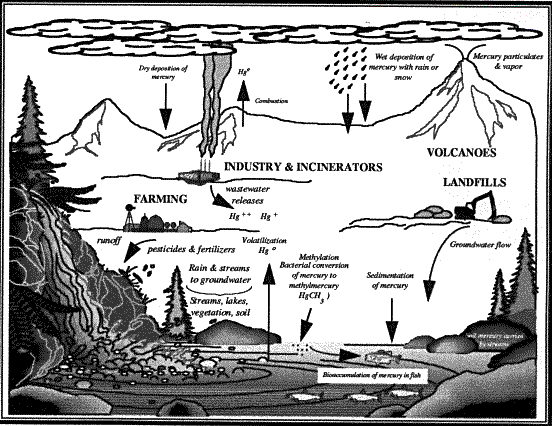 |
Diagram of how Hg spreads in our
environment
|